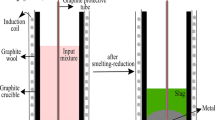
Abstract
Bauxite residue (BR) is an industrial waste from alumina refineries. Despite being comprised of valuable secondary materials, utilization of BR is limited owing to its high alkalinity and presence of some toxic constituents. Acid neutralization is widely used as a pre-treatment step to mitigate the problems caused by the alkalinity of BR. In this work, BR was treated with various concentrations of phosphoric acid (1 M, 3 M, 5 M, 8 M) followed by thermal treatment to recover the valuables (metals) in terms of metal phosphates. Phosphates of Al and Si were the main constituents of the residue while the supernatant contained mainly Fe and Al. The supernatant solution was acidic (pH between 1.5 and 1.9) and hence can be used to treat BR in place of phosphoric acid to obtain the metal phosphates as before. The 1 M sample residue showed presence of P2O5 like superphosphate fertilizer. The water leaching test showed insignificant levels of metal dissolution in the 8 M sample, thus exhibiting its ability toward immobilizing the constituents of BR. Additionally, the phosphates can be melted at 1000–1100 °C indicating their suitability for glass forming. Thus, phosphoric acid treatment of BR can result in phosphates that can be used in a range of applications.










Similar content being viewed by others
Data availability
Not applicable.
References
Evans K (2016) The history, challenges, and new developments in the management and use of bauxite residue. J Sust Metall 2:316–331. https://doi.org/10.1007/s40831-016-0060-x
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl 141:483–498. https://doi.org/10.1016/j.resconrec.2018.11.006
https://international-aluminium.org/resource/sustainable-bauxite-mining-guidelines-second-edition-2022-2/ (accessed on Jan 26/01/2023)
Power G, Gräfe M, Klauber C (2011) Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometall 108:33–45. https://doi.org/10.1016/j.hydromet.2011.02.006
Borra CR, Pontikes Y, Binnemans K, Gerven TV (2015) Leaching of rare earths from bauxite residue (red mud). Miner Engg 76:20–27. https://doi.org/10.1016/j.mineng.2015.01.005
Alkan G, Yagmurlu B, Gronen L, Dittrich C, Ma Y, Stopic S, Friedrich B (2019) Selective silica gel free scandium extraction from Iron-depleted red mud slags by dry digestion. Hydrometall 185:266–272. https://doi.org/10.1016/j.hydromet.2019.03.008
Hertel T, Van den Bulck A, Blanpain B, Pontikes Y (2020) An integrated process for iron recovery and binder production from bauxite residue (red mud). Mater Lett 264:127273. https://doi.org/10.1016/j.matlet.2019.127273
Deng B, Li G, Luo J, Ye Q, Liu M, Peng Z, Jiang T (2017) Enrichment of Sc2O3 and TiO2 from bauxite ore residues. J Haz Mater 331:71–80. https://doi.org/10.1016/j.jhazmat.2017.02.022
Wagh AS, Jeong SY (2003) Chemically bonded phosphate ceramics: III, reduction mechanism and its application to iron phosphate ceramics. J Am Ceram Soc 86:1850–1855. https://doi.org/10.1111/j.1151-916.2003.tb03571.x
Pepper RA, Couperthwaite SJ, Millar GJ (2016) Comprehensive examination of acid leaching behaviour of mineral phases from red mud: recovery of Fe, Al, Ti, and Si. Miner Engg 99:8–18. https://doi.org/10.1016/j.mineng.2016.09.012
Xue S, Li M, Jiang J, Millar GJ, Li C, Kong X (2019) Phosphogypsum stabilization of bauxite residue: conversion of its alkaline characteristics. J Environ Sci 77:1–10. https://doi.org/10.1016/j.jes.2018.05.016
Eastham J, Morald T (2006) Effective nutrient sources for plant growth on bauxite residue: II. Evaluating the response to inorganic fertilizers. Water Air Soil Pollut 171:315–331. https://doi.org/10.1007/s11270-005-9055-0
Summers R, Clarke M, Pope T, O’Dea T (2000) Comparison of single superphosphate and superphosphate coated with bauxite residue for subterranean clover production on phosphorus-leaching soils. Soil Res 38:735–744. https://doi.org/10.1071/SR99070
Haynes R, Zhou YF (2019) Natural ripening with subsequent additions of gypsum and organic matter is key to successful bauxite residue revegetation. J Cent South Univ 26:289–303. https://doi.org/10.1007/s11771-019-4001-2
Zeng H, Lyu F, Sun W, Zhang H, Wang L, Wang Y (2020) Progress on the industrial applications of red mud with a focus on China. Minerals 10:773. https://doi.org/10.3390/min10090773
Hu P, Zhang Y, Wang X, Zhou Y, Tong W, Luan X, Ma X, Zhou F, Chu PK, Zhao P (2018) K2MgSi3O8 in slow-release mineral fertilizer prepared by sintering of by-product of red mud-based flocculant. Environ Eng Sci 35:829–835. https://doi.org/10.1089/ees.2017.0340
Jones BEH, Haynes RJ, Phillips IR (2012) Cation and anion leaching and growth of Acacia saligna in bauxite residue sand amended with residue mud, poultry manure and phosphogypsum. Environ Sci Pollut Res 19:835–846. https://doi.org/10.1007/s11356-011-0630-1
Carlo ED, Boullemant A, Courtney R (2020) Ecotoxicological risk assessment of revegetated bauxite residue: implications for future rehabilitation programmes. Sci Total Environ 698:134344. https://doi.org/10.1016/j.scitotenv.2019.134344
Wagh AS, Jeong SY (2003) Chemically bonded phosphate ceramics: I, a dissolution model of formation. J Am Ceram Soc 86:1838–1844. https://doi.org/10.1111/j.1151-2916.2003.tb03569.x
O’connor G, Courtney R (2020) Constructed wetlands for the treatment of bauxite residue leachate: long term field evidence and implications for management. Ecol Eng 158:106076. https://doi.org/10.1016/j.ecoleng.2020.106076
Ghosh I, Guha S, Balasubramaniam R, Kumar AVR (2011) Leaching of metals from fresh and sintered red mud. J Haz Mater 185:662–668. https://doi.org/10.1016/j.jhazmat.2010.09.069
Bale CW, Bélisle E, Chartrand P, Decterov SA, Eriksson G, Gheribi AE, Hack K, Jung IH, Kang YB, Melançon J, Pelton AD, Petersen S, Robelin C, Sangster J, Spencer P, Van Ende M-A (2016) FactSage thermochemical software and databases—2010–2016. Calphad 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Liu Z, Li H (2015) Metallurgical process for valuable elements recovery from red mud—a review. Hydrometall 155:29–43. https://doi.org/10.1016/j.hydromet.2015.03.018
Gräfe M, Klauber C (2011) Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation. Hydrometall 108:46–59. https://doi.org/10.1016/j.hydromet.2011.02.005
Das B, Mohanty K (2019) A review on advances in sustainable energy production through various catalytic processes by using catalysts derived from waste red mud. Renew Energ 143:1791–1811. https://doi.org/10.1016/j.renene.2019.05.114
Rai S, Bahadure S, Chaddha MJ, Agnihotri A (2020) Disposal practices and utilization of red mud (Bauxite Residue): a review in Indian context and abroad. J Sust Metall 6:1–8. https://doi.org/10.1007/s40831-019-00247-5
Tsakanika LA, Panagiotatos G, Lymperopoulou T, Chatzitheodoridis E, Ochsenkühn K, Ochsenkühn-Petropoulou M (2022) Direct phosphoric acid leaching of bauxite residue for selective scandium extraction. Metals 12:228. https://doi.org/10.3390/met12020228
Li G, Ye Q, Deng B, Luo J, Rao M, Peng Z, Jiang T (2018) Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometall 176:62–68. https://doi.org/10.1016/j.hydromet.2018.01.007
Deng B, Li G, Luo J, Ye Q, Liu M, Rao M, Jiang T, Bauman L, Zhao B (2019) Selectively leaching the iron-removed bauxite residues with phosphoric acid for enrichment of rare earth elements. Sep Purif Technol 227:115714. https://doi.org/10.1016/j.seppur.2019.115714
Wagh AS, Jeong SY (2003) Chemically bonded phosphate ceramics: II, warm-temperature process for alumina ceramics. J Am Ceram Soc 86:1845–1849. https://doi.org/10.1111/j.1151-2916.2003.tb03570.x
Burke IT, Peacock CL, Lockwood C, Stewart DI, Mortimer RJG, Wards MB, Renforth P, Gruiz K, Mayes WM (2013) Behaviour of aluminium, arsenic and vanadium during the neutralization of red mud leachate by HCl, gypsum and seawater. Environ Sci Technol 47(12):6527–6535. https://doi.org/10.1021/es4010834
Higgins D, Curtin T, Pawlett M, Courtney R (2016) The potential for constructed wetlands to treat alkaline bauxite-residue leachate: Phragmites australis growth. Environ Sci Pollut Res 23:24305–24315. https://doi.org/10.1007/s11356-016-7702-1
Higgins D, Curtin T, Burke I, Courtney R (2018) The potential for constructed wetland mechanisms to treat alkaline bauxite residue leachate: carbonation and precipitate characterization. Environ Sci Pollut Res 25:29451–29458. https://doi.org/10.1007/s11356-018-2983-1
Higgins D, Curtin T, Courtney R (2017) Effectiveness of a constructed wetland for treating alkaline bauxite residue leachate: a 1-year field study. Environ Sci Pollut Res 24:8516–8524. https://doi.org/10.1007/s11356-017-8544-1
Acknowledgements
The authors would like to thank Hindalco (Belagavi) for providing the bauxite residue. We would also like to acknowledge the partial support received from Department of Science and Technology (DST), Govt. of India, through the Grant No. EEQ/2016/000276.
Novelty statement
A simple method was used to recover useful resources from bauxite residue (BR), which is an industrial waste. The method involved phosphoric acid treatment which converted BR into metal phosphates which can be used for various applications such as glass making and fertilizers. It was also demonstrated by a water leaching test that the constituents of the phosphates can be immobilized by the used process thus making them stable and safe to the environment.
Funding
No funding to declare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. RB conceptualized the idea, provided guidance throughout the work and contributed in manuscript writing. KKM carried out all experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mayuranathan, K.K., Bauri, R. A facile method of resource recovery from bauxite residue by phosphoric acid treatment. J Mater Cycles Waste Manag 25, 2146–2158 (2023). https://doi.org/10.1007/s10163-023-01668-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01668-x