Abstract
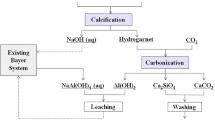
The production of one ton of alumina using the Bayer process generates 0.7–2.0 ton of Bauxite Residue (BR) and an average of 1.0 ton of CO2. The direct use of exhaust gases to react and reduce the alkalinity of BR may allow a triple gain: improving the storage conditions, opening a range of new applications for BR and sequester from 16 to 102 kg of CO2 per ton of alumina. This paper shows a lab scale long term program to measure the effects of adding different percentages of Ca and Mg hydroxides followed by carbonation in order to enhance the precipitation of the alkalinity on stable compounds. Analysis by X-ray diffraction and scanning electron microscopy was done to monitor the appearance of carbonates. The BR pH was monitored for 600 days to evaluate the buffer effect and compared with the stabilization pH of carbonated bauxite residue without addition.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Evans, K. Management and use of bauxite residue. Aluminium International Today, v. 28, n. 2, p. 19, 2016.
Wanchao L; et al, New Progresses on Utilization of Red Mud in China, Icsoba newsletter, Vol 15, Jun 2016, http://www.icsoba.org/newsletters.
Alumina Technology Roadmap –2010, http://www.world-aluminium.org/media/filer/2012/06/12/fl0000422.pdf.
Venancio, L. C.; Souza, J. A. S.; et al., Pilot Test of Bauxite Residue Carbonation With Flue Gas, Light Metals, TMS 2013, San Antonio.
Venancio, L. C.; Souza, J. A. S.; et al., Bauxite Residue Amendment Through the Addition of Ca and or Mg Followed by Carbonation, Light Metals, TMS 2017, San Diego.
Nikraz, H. R.; Bodley, A. J.; Cooling, D. J.; Kong, P. Y. L.; Soomro, M.; 2007, Comparison of Physical Properties between Treated and Untreated Bauxite Residue Mud, Journal of Materials in Civil Engineering, ASCE/January 2007.
Khaitan, S., Dzombak, D. A., Lowry, G. V., “Mechanisms of neutralization of bauxite residue by carbon dioxide”, Journal of Environmental Engineering, ASCE, 2009.
Gräfe, M.; Power, G., Klauber, C., 2009, Review of bauxite residue alkalinity and associated chemistry; DMR-3610; CSIRO-Minerals.
Thornber, M. R.; Binet, D., Caustic soda adsorption on Bayer residues. In 5th International Alumina Quality Workshop, Ed. AQW Inc.: Bunbury, 1999; pp 498–507.
Cooling, D. J.; Hay, P. S.; Guilfoyle, L., Carbonation of bauxite residue, Proceedings of the 6th International Alumina Quality Workshop, Chandrashekar, S., Ed. AQW Inc., Brisbane; pp 185–190, 2002.
Johnston, M., Clark, M.W., McMahon, P. et al., Alkalinity Conversion of Bauxite Refinery Residues by Neutralization, Journal of Hazardous Materials 182, Elsevier, 2010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Venancio, L.C.A. et al. (2018). Analyzing the Bauxite Residue Amendment Through the Addition of Ca and Mg Hydroxides Followed by Carbonation. In: Martin, O. (eds) Light Metals 2018. TMS 2018. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-72284-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-72284-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72283-2
Online ISBN: 978-3-319-72284-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)