Abstract
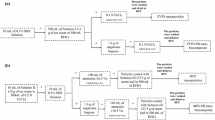
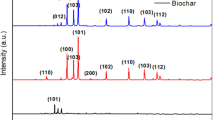
The preparation of biochar from Tannery (chrome) sludge has gained recent attention among many scientists globally. Hydrothermal pre-treatment method was adopted to load TiO2 nanoparticles on the surface of sludge biochar to develop a safe, low-cost, and novel nanocomposite. Chemical activation of sludge with KOH solution for 48 h was done before pyrolyzing the sludge to produce biochar. This novel c-SB/TiO2 nanocomposite exhibits a higher surface area of 636.23 m2/g when compared with the sludge biochar/TiO2 nanocomposite without chemical activation. The present research work mainly focuses on the synthesis and investigation of structural properties of c-SB/TiO2 nanocomposite prepared by hydrothermal pre-treatment. The morphological and structural properties of sludge biochar/TiO2 nanocomposite were identified by Scanning Electron Microscope (SEM) and X-ray Diffraction (XRD). The porosity of the nanocomposite was investigated using a BET surface area analyser. The Thermal decomposition and surface functional group was identified using TGA, FT-IR, PL and Raman spectrophotometer, respectively. c-SB/TiO2 nanocomposite was used to degrade Cr (VI) by varying its initial concentration, catalyst dosage and initial pH. Results indicate that c-SB/TiO2 nanocomposite shows an enhanced photocatalytic activity under UV light irradiation. Further, it was found that under optimal conditions of 10 mg/L of pollutant concentration, pH of 2 and catalyst dosage of 0.3 g/L, degradation efficiency of 98.65% Cr (VI) has been achieved for a reaction time of 180 min. Additionally, COD measurement shows a 69.4% reduction for a reaction time of 180 min, which is found to be more recalcitrant under photocatalytic activity.
Graphical abstract














Similar content being viewed by others
References
Patnaik R (2018) Impact of industrialization on environment and sustainable solutions—reflections from a South Indian Region. IOP Conf Ser: Earth Environ Sci 120:012016
Chandrasekaran A et al (2015) Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India—spectroscopical approach. Spectrochim Acta Part A Mol Biomol Spectrosc 137:589–600
Barbosa RFS et al (2020) Chromium removal from contaminated wastewaters using biodegradable membranes containing cellulose nanostructures. Chem Eng J 395:125055
Khademi H et al (2019) Environmental impact assessment of industrial activities on heavy metals distribution in street dust and soil. Chemosphere 217:695–705
Song Z, Williams CJ, Edyvean RGJ (2000) Sedimentation of tannery wastewater. Water Res 34(7):2171–2176
Reemtsma T, Jekel M (1997) Dissolved organics in tannery wastewaters and their alteration by a combined anaerobic and aerobic treatment. Water Res 31(5):1035–1046
Kotaś J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107(3):263–283
Zhitkovich A (2011) Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol 24(10):1617–1629
Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13:217
Egodawatte S et al (2015) Chemical insight into the adsorption of chromium (III) on iron oxide/mesoporous silica nanocomposites. Langmuir 31(27):7553–7562
Owlad M et al (2009) Removal of hexavalent chromium-contaminated water and wastewater: a review. Water Air Soil Pollut 200(1):59–77
Wei L et al (2020) Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ Int 144:106093
Bian Y et al (2018) Recycling of waste sludge: preparation and application of sludge-based activated carbon. Int J Polym Sci 2018:8320609
Almahbashi NMY et al (2021) Optimization of preparation conditions of sewage sludge based activated carbon. Ain Shams Eng J 12(2):1175–1182
Xu G, Yang X, Spinosa L (2015) Development of sludge-based adsorbents: preparation, characterization, utilization and its feasibility assessment. J Environ Manage 151:221–232
Devi P, Saroha AK (2017) Utilization of sludge based adsorbents for the removal of various pollutants: a review. Sci Total Environ 578:16–33
Hii K et al (2014) A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Biores Technol 155:289–299
Jeon E-K et al (2018) Enhanced adsorption of arsenic onto alum sludge modified by calcination. J Clean Prod 176:54–62
Zhang L et al (2019) Combined physical and chemical activation of sludge-based adsorbent enhances Cr(VI) removal from wastewater. J Clean Prod 238:117904
Gupta R et al (2022) Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J Mater Cycles Waste Manage 24(3):852–876
Wang X et al (2009) Preparation of activated carbons from wet activated sludge by direct chemical activation. Water Sci Technol 59(12):2387–2394
Huang H et al (2007) Production and characterization of KOH-activated carbon derived from polychlorinated biphenyl residue generated by the sodium dispersion process. J Mater Cycles Waste Manage 9(2):182–187
Malhotra M, Garg A (2017) Hydrothermal pretreatment of waste activated sludge (WAS), National Conference on Sustainable Advanced Technologies for Environmental Management (SATEM-2017), Paper-15
Chen T et al (2014) Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Biores Technol 164:47–54
Chen T et al (2015) Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Biores Technol 190:388–394
Sun H et al (2015) Potential of sludge carbon as new granular electrodes for degradation of acid orange 7. Ind Eng Chem Res 54(20):5468–5474
Xin W, Li X, Song Y (2020) Sludge-based mesoporous activated carbon: the effect of hydrothermal pretreatment on material preparation and adsorption properties of bisphenol A (BPA). J Chem Technol Biotechnol 95:1666
Vijayan R et al (2018) Removal of toxic pollutants using tannery sludge derived mesoporous activated carbon: experimental and modelling studies. J Environ Chem Eng 7:102798
Yu Z-W et al (2019) Production of activated carbon from sludge and herb residue of traditional Chinese medicine industry and its application for methylene blue removal. BioResources. https://doi.org/10.15376/biores.14.1.1333-1346
Park S et al (2020) Effect of hydrothermal pre-treatment on physical properties and co-digestion from food waste and sewage sludge mixture. Waste Manage Res 38(5):546–553
Wang ZW et al (2016) Comprehensive evaluation of the liquid fraction during the hydrothermal treatment of rapeseed straw. Biotechnol Biofuels 9:142
Qutub N et al (2016) Synthesis of CdS nanoparticles using different sulfide ion precursors: formation mechanism and photocatalytic degradation of acid blue-29. J Environ Chem Eng 4(1):808–817
Sun J et al (2006) Photocatalytic degradation and kinetics of Orange G using nano-sized Sn(IV)/TiO2/AC photocatalyst. J Mol Catal A 260(1):241–246
Theron J, Walker JA, Cloete TE (2008) Nanotechnology and water treatment: applications and emerging opportunities. Crit Rev Microbiol 34(1):43–69
Yan M et al (2017) Effect of inorganic coagulant addition under hydrothermal treatment on the dewatering performance of excess sludge with various dewatering conditions. J Mater Cycles Waste Manage 19(3):1279–1287
Jain A, Balasubramanian R, Srinivasan MP (2016) Hydrothermal conversion of biomass waste to activated carbon with high porosity: a review. Chem Eng J 283:789–805
Kiliç E et al (2011) Chromium recovery from tannery sludge with saponin and oxidative remediation. J Hazard Mater 185(1):456–462
Tasca AL et al (2020) Investigating the activation of hydrochar from sewage sludge for the removal of terbuthylazine from aqueous solutions. J Mater Cycles Waste Manage 22(5):1539–1551
Vijayan R et al (2018) Activated carbon (prepared from secondary sludge biomass) supported semiconductor zinc oxide nanocomposite photocatalyst for reduction of Cr(VI) under visible light irradiation. J Environ Chem Eng 6:7327
Pawar V et al (2019) Influence of synthesis route on structural, optical, and electrical properties of TiO2. Appl Phys A. https://doi.org/10.1007/s00339-019-2948-3
Phoungthong K et al (2018) Leaching characteristics and phytotoxic effects of sewage sludge biochar. J Mater Cycles Waste Manage 20(4):2089–2099
Ncibi MC et al (2009) Preparation and characterisation of raw chars and physically activated carbons derived from marine Posidonia oceanica (L.) fibres. J Hazard Mater 165(1–3):240–249
Chen W et al (2019) Porosity and surface chemistry development and thermal degradation of textile waste jute during recycling as activated carbon. J Mater Cycles Waste Manage 21(2):315–325
Wang W, Chen W-H, Jang M-F (2020) Characterization of hydrochar produced by hydrothermal carbonization of organic sludge. Future Cities Environ. https://doi.org/10.5334/fce.102
Wang D et al (2018) Removal of nitrobenzene from aqueous solution by adsorption onto carbonized sugarcane bagasse. Adsorpt Sci Technol 36:026361741877182
Sane P et al (2018) Photocatalytic reduction of chromium (VI) using combustion synthesized TiO2. J Environ Chem Eng 6(1):68–73
Spurr RA, Myers H (1957) Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal Chem 29(5):760–762
Li G et al (2010) Effect of the agglomeration of TiO2 nanoparticles on their photocatalytic performance in the aqueous phase. J Colloid Interface Sci 348(2):342–347
Nasikhudin et al (2018) Study on photocatalytic properties of TiO2 nanoparticle in various pH condition. In: Journal of physics: conference series, Vol 1011. No 1, 012069
Zhao Y-X et al (2020) Production of carbon-doped titanium dioxide (C–TiO2) from polytitanium-coagulated sludge as an adsorbent or photocatalyst for pollutant removals. J Clean Prod 267:121979
Wang L et al (2008) Photocatalytic reduction of Cr(VI) over different TiO2 photocatalysts and the effects of dissolved organic species. J Hazard Mater 152(1):93–99
Payel S, Hashem MA, Hasan MA (2021) Recycling biochar derived from tannery liming sludge for chromium adsorption in static and dynamic conditions. Environ Technol Innov 24:102010
Villalobos-Lara AD et al (2021) Electrocoagulation treatment of industrial tannery wastewater employing a modified rotating cylinder electrode reactor. Chemosphere 264:128491
Scrimieri L et al (2020) Enhanced adsorption capacity of porous titanium dioxide nanoparticles synthetized in alkaline sol. Appl Phys A 126(12):926
Sharafinia S, Farrokhnia A, GhasemianLemraski E (2021) Comparative study of adsorption of safranin o by TiO2/activated carbon and chitosan/TiO2/activated carbon adsorbents. Phys Chem Res 9:605–621
Masoudian N, Rajabi M, Ghaedi M (2019) Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of Congo red and phenol red dyes from wastewaters. Polyhedron 173:114105
Anjum M et al (2019) Remediation of wastewater using various nano-materials. Arab J Chem 12(8):4897–4919
Singh G et al (2013) ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon 50:385–394
Duan X et al (2015) Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl Mater Interfaces 7(7):4169–4178
Peng H et al (2017) Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ Pollut 229:846–853
Zhang H et al (2017) Microwave pyrolysis of textile dyeing sludge in a continuously operated auger reactor: char characterization and analysis. J Hazard Mater 334:112–120
Ku Y, Jung I-L (2001) Photocatalytic reduction of Cr (VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35(1):135–142
Ramasamy B, Jeyadharmarajan J, Chinnaiyan P (2021) Novel organic assisted Ag-ZnO photocatalyst for atenolol and acetaminophen photocatalytic degradation under visible radiation: performance and reaction mechanism. Environ Sci Pollut Res 28(29):39637–39647
Acknowledgements
We would like to express our gratitude to TEQIP III CoE-ES for providing us with all the facilities and equipment required to carry out our research work in a successful way.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Velumani, M., Jeyadharmarajan, J. Recycling of Tannery (chrome) sludge into sludge biochar (SB) /TiO2 nanocomposite via chemical activation through hydrothermal pre-treatment. J Mater Cycles Waste Manag 24, 2255–2269 (2022). https://doi.org/10.1007/s10163-022-01483-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-022-01483-w