Abstract
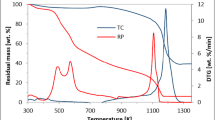
Large-area stacking of solid waste phosphogypsum (PG) and coal gangue (CG) poses environmental contamination hazards. This work presents the reaction characteristics, thermal decomposition behavior and reaction kinetics of composite reducing agent on PG decomposition. The results show that the composite reducing agent of CG and coke can efficiently decompose PG. The optimal mass ratio is CG:coke:PG = 1:2:10, at which the decomposition rate reaches more than 99%, and the solid products are CaS, Ca2Al2SiO7, etc. The initial decomposition temperature of PG decomposition by composite reducing agent is 815 ℃, while it is 860 ℃ by coke. The average apparent activation energy of PG decomposition with composite reducing agent is lower than that with coke, which was calculated by Flynn–Wall–Ozawa (FWO) and Kissinger–Akahira–Sunose (KAS). The synergistic effect of CG and coke clearly decrease the temperature and improve the reactivity of PG decomposition. This work provides an efficient method to cooperatively utilize of two kinds of industrial solid wastes via composite reducing agent decomposes PG.










Similar content being viewed by others
References
Cui SF, Fu YZ, Zhou BQ, Li JX, He WY, Yu YQ, Yang JY (2021) Transfer characteristic of fluorine from atmospheric dry deposition, fertilizers, pesticides, and phosphogypsum into soil. Chemosphere 278:130432. https://doi.org/10.1016/j.chemosphere.2021.130432
Chernysh Y, Yakhnenko O, Cgubur V, Roubik H (2021) Phosphogypsum recycling: a review of environmental issues, current trends, and prospects. Appl Sci 11(4):1575. https://doi.org/10.3390/app11041575
Kuzmanović P, Todorović N, Mrđa D, Forkapić S, Petrović LF, Miljević B, Jan Hansman J, Knežević J (2021) The possibility of the phosphogypsum use in the production of brick: Radiological and structural characterization. J Hazard Mater 413:125343. https://doi.org/10.1016/j.jhazmat.2021.125343
Wang Y, Wan TM, Zhong YJ, Ma XD, Chen ZY, Wang XL (2020) Environmental sustainability of renewable phosphogypsum by CaS. J Therm Anal Calorim 139:3457–3471. https://doi.org/10.1007/s10973-019-08718-3
Wu S, Yao YG, Yao XL, Ren CZ, Li JW, Xu D, Wang WL (2020) Co-preparation of calcium sulfaluminate cement and sulfuric acid through mass utilization of industrial by-product gypsum. J Clean Prod 265:121801. https://doi.org/10.1016/j.jclepro.2020.121801
Rekha S, Anila EI (2019) Intense yellow emitting biocompatible CaS: Eu nanophosphors synthesized by wet chemical method. J Fluorsec 29(3):673–683. https://doi.org/10.1007/s10895-019-02375-3
Yang HF, Xu YT, Shen K, Qiu YH, Zhang G (2018) Removal of heavy metal ions from zine hydrometallurgical wastewater using CaS-containing alkaline slag. J Environ Chem Eng 6:6451–6456. https://doi.org/10.1016/j.jece.2018.09.040
Antar K, Jemal M (2018) A thermogravimetric study into the effects of additives and water vapor on the reduction of gypsum and Tunisian phosphogypsum with graphite or coke in a nitrogen atmosphere. J Therm Anal Calorim 132:113–125. https://doi.org/10.1007/s10973-017-6871-6
Zhu LP, Zheng SC (2019) Experimental study of preparing CaS from phosphogypsum with lignite. IOP Conf Ser: Earth Environ Sci 267:032077. https://doi.org/10.1088/1755-1315/267/3/032077
Zheng SC, Ning P, Ma LP, Niu XK, Zhang W, Chen YH (2011) Reductive decomposition of phosphogypsum with high-sulfur-concentration coal to SO2 in an inert atmosphere. Chem Eng Res Des 89:2736–2741. https://doi.org/10.1016/j.cherd.2011.03.016
Zheng SC, Ning P, Ma LP, Chen FX, Shi JY (2014) Phosphogypsum as a raw material for the production of SO2 and lime in circulating fluidized beds. Combust Sci Technol 186:377–386. https://doi.org/10.1080/00102202.2013.864286
Yan ZQ, Wang ZA, Liu H, Tu YJ, Yang W, Zeng HC, Qiu JR (2015) Decomposition and solid reactions of calcium sulfate doped with SiO2, Fe2O3 and Al2O3. J Anal Appl Pyrolysis 113:491–498. https://doi.org/10.1016/j.jaap.2015.03.019
Fang H, Xie RS (2019) Phosphogypsum pyrolysis with mineralization agent under weak reducing atmosphere. IOP Conf Ser: Earth Environ Sci 295:052030. https://doi.org/10.1088/1755-1315/295/5/052030
Lu DH, Chen QL, Li CQ, Gong S (2021) Effect of potassium feldspar on the decomposition rate of phosphogypsum. J Chem Technol Biotechnol 96:374–383. https://doi.org/10.1002/jctb.6549
Zhao LJ, Wan TM, Yang XS, Yang L, Kong XJ, Zhang ZY, Wang XL (2015) Effects of kaolinite addition on the melting characteristics of the reaction between phosphogypsum and CaS. J Therm Anal Calorim 119:2119–2126. https://doi.org/10.1007/s10973-015-4400-z
Antar K, Jemal M (2021) Recovery of calcium sulfide from Tunisian phosphogypsum and olive mill wastewater: a thermogravimetric and kinetic study. J Therm Anal Calorim. https://doi.org/10.1007/s10973-021-10773-8
Suslikov AV, Zhirnov BS, Murtazin FR (2021) A study of the kinetics of reaction petroleum coke with phosphogypsum to give calcium sulfide. Chem Technol Fuels Oils 57(3):461–466. https://doi.org/10.1007/s10553-021-01266-3
Zhang YL, Ling TC (2020) Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—a review. Constr Build Mater 234:117424. https://doi.org/10.1016/j.conbuildmat.2019.117424
Huang M, Duan JM, Wang JK (2021) Research on basic mechanism properties and fracture damage of coal gangue concrete subjected to freeze-thaw cycles. Adv Mater Sci Eng 368:112–124. https://doi.org/10.1155/2021/6701628
Ma HQ, Zhu HG, Wu C, Chen HY, Sun JW, Liu JY (2020) Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol 368:112–124. https://doi.org/10.1016/j.powtec.2020.04.054
Zhang YY, Gou YX, Cheng FQ, Yan KZ, Cao Y (2015) Investigation of combustion characteristic and kinetic of coal gangue with different feedstock properties by thermogravimetric analysis. Thermochim Acta 614:137–148. https://doi.org/10.1016/j.tca.2015.06.018
Xie T, Wei RC, Wang Z, Wang J (2020) Comparative analysis of thermal oxidative decomposition and fire characteristics for different straw powders via thermogravimetry and cone calorimetry. Process Saf Environ Prot 134:121–130. https://doi.org/10.1016/j.psep.2019.11.028
Chaalal O, Madhuranthakam CMR, Moussa B, Hosssain MM (2020) Sustainable approach for recovery of sulfur from phosphogypsum. ACS Omega 5:8151–8157. https://doi.org/10.1021/acsomega.0c00420
Shi T, Wan TM, Zhang ZY, Yang XS, Yang L, Zhong BH, Kong XJ, Wang XL (2016) Effect of SiO2 on the melting characteristics of reaction between phosphogypsum and calcium sulfide. J Therm Anal Calorim 123:1601–1609. https://doi.org/10.1007/s10973-015-5032-z
Alla M, Hafiany ML, Gharibi EK, Ghalit M (2019) Thermodynamic study of the desulfurization process of phosphogypsum. Mater Today: Proc 13:556–561. https://doi.org/10.1016/j.matpr.2019.04.013
Cao CY, Liu HM, Zhang D, Zhu K, Li AJ, Wang LL, Hu HY (2021) Investigation on the decomposition mechanism and kinetic behavior of 5-aminotetrazole with metal oxide produced by added coolants. Fuel 303:121315. https://doi.org/10.1016/j.fuel.2021.121215
Sathiva PSP, Swaminathan G, Viraj VJ (2021) Thermogravimetric analysis of hazardous waste: pet-coke, by kinetic models and artificial neural network modeling. Fuel 287:119470. https://doi.org/10.1016/j.fuel.2020.119470
Yan JC, Jiao HR, Li ZK, Lei ZP, Wang ZC, Ren SB, Shui HF, Kang SG, Yan HL, Pan CX (2019) Kinetic analysis and modeling of coal pyrolysis with model-free methods. Fuel 241:382–391. https://doi.org/10.1016/j.fuel.2018.12.079
Huynh TTT, Ma TC, Dang CH, Vo TTT, Nguyen DT, Dang VS, Nguyen KDV, Tran VT, Nguyen TD (2020) Influence of extractions on physicochemical characterization and bioactivity of Piper nigrum oils: study on the non-isothermal decomposition kinetic. Arab J Chem 13:7289–7301. https://doi.org/10.1016/j.arabjc.2020.08.008
Burkeev M, Fazylov S, Bakirova R, Sarsenbekova A, Tazhbaey E, Davrenbekov S (2021) Thermal decomposition of β-cyclodextrin and its inclusion complex with vitamin E. Mendeleev Commum 31:76–78. https://doi.org/10.1177/0734242X17748362
Zheng DL, Ma LP, Wang RM, Yang J, Dai QX (2018) Decomposing properties of phosphogypsum with iron addition under two-step cycle multi-atmosphere control in fluidised bed. Waste Manag Res 36(2):183–193. https://doi.org/10.1177/0734242X17748362
Julphunthong P (2018) Synthesizing of calcium sulfoaluminate-belite (CSAB) cements from industrial waste materials. Mater Today: Proc 5(7):14933–14938. https://doi.org/10.1016/j.matpr.2018.04.033
Nidheesh PV, Suresh Kumar M (2019) An overview of environmental sustainability in cement and steel production. J Clean Prod 231:856–871. https://doi.org/10.1016/j.jclepro.2019.05.251
Huang GJ, Lin JM, Yang B, Li MD, Li YJ, Zhai W, Wang ZG, Li GB, Li LS (2018) Comparative thermal decomposition kinetic analysis of the biodegradable terpolymer poly (lactide-co-propylene carbonate) applied by various theoretical models. Polym Test 71:95–100. https://doi.org/10.1016/j.polymertesting.2018.08.025
Ma J, Luo HN, Li Y, Liu ZG, Li D, Gai C, Jiao WT (2019) Pyrolysis kinetics and thermodynamic parameters of the hydrochars derived from co-hydrothermal carbonization of sawdust and sewage sludge using thermogravimetric analysis. Bioresour Technol 282:133–141. https://doi.org/10.1016/j.biortech.2019.03.007
Galwey AK (2003) What is meant by the term variable activation energy’ when applied in the kinetic analyses of solid-state decompositions (crystolysis reactions)? Thermochim Acta 397(1–2):249–268. https://doi.org/10.1016/S0040-6031(02)00271-X
Santos VO, Queiroz LS, Araujo RO, Ribeiro FCP, Guimarães MN, Costa CEF, Chaar JS, Souza KC (2020) Pyrolysis of acai seed biomass: kinetics and thermodynamic parameters using thermogravimetric analysis. Bioresour Technol Rep 12:100553. https://doi.org/10.1016/j.biteb.2020.100553
Sobek S, Werle S (2020) Insoconversional determination of the apparent reaction models governing pyrolysis of wood, straw and sewage sludge, eith an approach to rate modelling. Renewable Energy 161:972–987. https://doi.org/10.1016/j.renene.2020.07.112
Cen P, Bian X, Wu WY (2020) Isoconversional kinetic analysis of decomposition of bastnaesite concentrates with calcium hydroxide. J Rare Earths 38:1361–1371. https://doi.org/10.1016/j.jre.2020.01.006
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110(35):17315–17328. https://doi.org/10.1021/jp062746a
Tian HJ, Guo QJ, Chang J (2008) Investigation into decomposition behavior of CaSO4 in chemical-looping combustion. Energy Fuels 22:3915–3921. https://doi.org/10.1021/ef800508w
Acknowledgements
This work was supported by the National Key Research and Development Program (No. 2018YFC1903500), the Major Science and Technology Project in Guizhou Province (No. [2019]5410), and the Top Hundred Talents of Science and Technology Program of Guizhou Province (No. [2016]5658).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this manuscript have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Q., Ao, X., Chen, Q. et al. Reaction characteristics and kinetics of phosphogypsum decomposition via synergistic reduction effect of composite reducing agent. J Mater Cycles Waste Manag 24, 595–605 (2022). https://doi.org/10.1007/s10163-021-01344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-021-01344-y