Abstract
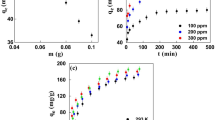
The aim of the present study was to analytically provide adsorption characteristics of Cu2+ and Zn2+ using carbonized food waste (CFW); more specifically, batch tests were conducted using various concentrations of metal ions, contact times, and initial pH levels in an attempt to understand the adsorption removal of heavy metal ions in aqueous solution at concentrations ranging between 50 and 800 mg/l. The results confirmed that the adsorption equilibrium was established within a maximum of 80 min, and the maximum concentrations for adsorption of Cu2+ and Zn2+ were 28.3 and 23.5 mg/g, respectively. These adsorption levels indicate that CFW has better performance than many other adsorbents. In experiments using different pH conditions, the applicability to acid wastewater was found to be high, and an excellent adsorption removal ratio of 75%–90% was observed under acid conditions at pH 2–4. Furthermore, as the adsorption time increased, the calcium component in the CFW began to leach into the aqueous solution and raise the pH, accordingly causing the removal of heavy metal ions partially as a result of precipitation. When our results were analyzed using the Langmuir model and the Freundlich model for isothermal adsorptivity, the activity of CFW in this study was shown to be more consistent with the former; the adsorption speed of Cu2+ and Zn2+ according to a pseudosecond-order reaction model was found to be very fast for an initial concentration of not more than 100 mg/l. In a test in which an attempt was made to compare adsorption capacity values obtained from the experiments in this study with the aforementioned three models, the pseudosecond-order reaction model was found to provide results closest to the actual values.
Similar content being viewed by others
References
Ross SM (1994) Toxic metals in soil-plant system. Wiley, New York
Klaassen CD (1986) Toxicology. McMillan, New York Publishing company
Ahmet S, Mustafa T (2007) Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater 149:283–291
Schlimm C, Heitz E (1996) Development of a wastewater treatment process: reduction dehalogenation of chlorinated hydrocarbons by metal. Environ Prog 15:38–47
Park KH, Park MA, Jang H, Kim EK, Kin YH (1996) Removal of heavy metals, cadmium II and lead II ions in water by sargassum. Anal Sci Technol 12:196–202
Petruzzelli D, Pagano M, Rriavanti G, Passino R (1999) Lead removal and recovery from battery waste waters by natural zeolite clinptilolite. Solvent Extr Ion Exch 17:677–694
Faghihian H, Ghannadi-Maragh M, Kazemian H (1999) The use of clinptilolite on radioactive cesium and strontium from nuclear waste water and lead, nickel, cadmium, barium from municipal waste water. Sep Sci Technol 34:2275–2292
Vecchio A, Carlo F, Damiano DS, Vincenza A (1998) Heavy metal biosorption by bacterial cells. Fresenius J Anal Chem 361:338–342
Sameer AA, Fawzi B, Fadel M (1999) Sorption of copper and nickel by spent animal bones. Chemosphere 39:2087–2096
Qi YM, Samuel JT, Terry JL (1993) In situ lead immobibization by apatite. J Environ Sci Technol 27:1803–1810
Hwang AN, Ji HW, Kim JY (2006) Characteristics of phosphorus containing waste bones. Mater Lett 61:677–679
Jiang MQ, Wang QP, Jin ZY, Chen ZL (2009) Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. J Hazard Mater 170:332–339
Ministry of Environment (Korea) (2004) The casebook of disposal and best facility for food waste. The Ministry, Gwacheon
Korea Food Research Institute (2002) Results for research outsourcing on economic value assessment of food resource thrown away as food wastes. The Institute, Sungnam
Han JG, Hong KK, Kim YW, Lee JY (2010) Enhanced electrokinetic (E/K) remediation on copper contaminated soil by CFW (carbonized foods waste). J Hazard Mater 177:530–538
Unuabonah EI, Adebowale KO (2008) Adsorption of Pb(II) and Cd(II) from aqueous solutions onto sodium tetraborate-modified kaolinite clay: equilibrium and thermodynamic studies. Hydrometallurgy 93:1–9
Kim YW, Lee JY, Lee YK, Han JG (2009) A study on heavy metals sedimentation by pH. Korea Society of Civil Engineering Annual Conference, gangwon, 2031–2034
Ören AH, Kayab A (2006) Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J Hazard Mater 131:59–65
Ahmad AH, Ribhi EB (1997) Removal of lead and nickel ions using zeolite tuff. J Chem Tech Biotechnol 69:27–34
Gupta BS, Curran M, Hasan S, Ghosh TK (2009) Adsorption characteristics of Cu and Ni on Irish peat moss. J Environ Manag 90:954–960
Prasad RK, Srivastava SN (2009) Sorption of distillery spent wash onto fly ash: kinetics, mechanism, process design and factorial design. J Hazard Mater 161:1313–1322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, JG., Lee, JY., Hong, KK. et al. Adsorption characteristics of Cu2+ and Zn2+ from aqueous solution using carbonized food waste. J Mater Cycles Waste Manag 12, 227–234 (2010). https://doi.org/10.1007/s10163-010-0292-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-010-0292-y