Abstract
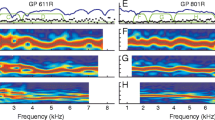
According to coherent reflection theory, otoacoustic emissions (OAE) evoked with clicks (clicked-evoked, CE) or tones (stimulus frequency, SF) originate via the same mechanism. We test this hypothesis in gerbils by investigating the similarity of CE- and SFOAEs across a wide range of stimulus levels. The results show that OAE transfer functions measured in response to clicks and sweeps have nearly equivalent time–frequency characteristics, particularly at low stimulus levels. At high stimulus levels, the two OAE types are more dissimilar, reflecting the different dynamic properties of the evoking stimulus. At mid to high stimulus levels, time–frequency analysis reveals contributions from at least two OAE source components of varying latencies. Interference between these components explains the emergence of strong spectral microstructure. Time–frequency filtering based on mean basilar-membrane (BM) group delays (τBM) shows that late-latency OAE components (latency ~ 1.6τBM) dominate at low stimulus intensities and exhibit highly compressive growth with increasing stimulus intensity. In contrast, early-latency OAE components (~ 0.7τBM) are small at low stimulus levels but can come to dominate the overall response at higher intensities. Although the properties of long-latency OAEs are consistent with an origin via coherent reflection near the peak of the traveling wave, the generation place and/or mechanisms responsible for the early-latency OAE components warrant further investigation. Because their delay remains in constant proportion to τBM across sound intensity, long-latency OAEs, whether evoked with tones or clicks, can be used to predict characteristics of cochlear processing, such as the sharpness of frequency tuning, even at high stimulus levels.











Similar content being viewed by others
References
Abdala C, Guardia YC, Shera CA (2018) Swept-tone stimulus-frequency otoacoustic emissions: Normative data and methodological considerations. J Acoust Soc Am 143:181
Altoè A, Shera CA (2020) Nonlinear cochlear mechanics without direct vibration-amplification feedback. Phys Rev Research 2:013218
Bennett CL, Ozdamar O (2010) Swept-tone transient-evoked otoacoustic emissions. J Acoust Soc Am 128:1833–1844
Charaziak KK, Siegel JH (2015a) Low-frequency tone-pip-evoked otoacoustic emissions originate over a broad cochlear region in chinchillas. In: Mechanics of hearing: protein to perception (Karavitaki KD, Corey DP, eds), p 090016
Charaziak KK, Siegel JH (2015b) Tuning of SFOAEs evoked by low-frequency tones is not compatible with localized emission generation. J Assoc Res Otolaryngol 16:317–329
Charaziak KK, Shera CA (2017) Compensating for ear-canal acoustics when measuring otoacoustic emissions. J Acoust Soc Am 141:515–531
Charaziak KK, Dong W, Shera CA (2018) Temporal interactions in basilar-membrane and otoacoustic-emission responses to pairs of clicks. Assoc Res Otolaryngol, Abstr: PS-465 41:296
Charaziak KK, Altoè A, Dong W, Shera CA (2019) Ringing in basilar-membrane responses to clicks - Effect on the tonotopic map. Assoc Res Otolaryngol, Abstr: PS-174 42:99
Charaziak KK, Altoé A, Oghalai J, Shera C (2020a) Measuring cochlear impulse responses using frequency sweeps. Assoc Res Otolaryngol, Abstr: PS-197 43
Charaziak KK, Dong W, Altoe A, Shera CA (2020b) Asymmetry and microstructure of temporal-suppression patterns in basilar-membrane responses to clicks: Relation to tonal suppression and traveling-wave dispersion. J Assoc Res Otolaryngol 21:151–170
Choi YS, Lee SY, Parham K, Neely ST, Kim DO (2008) Stimulus-frequency otoacoustic emission: Measurements in humans and simulations with an active cochlear model. J Acoust Soc Am 123:2651–2669
Clark CW, Marler P, Beeman K (1987) Quantitative analysis of animal vocal phonology: An application to swamp sparrow song. Ethology 76:101–115
Cooper NP, Vavakou A, van der Heijden M (2018) Vibration hotspots reveal longitudinal funneling of sound-evoked motion in the mammalian cochlea. Nat Commun 9:3054
de Boer E (1997) Connecting frequency selectivity and nonlinear models of the cochlea. Aud Neurosci 3:377–388
de Boer E, Nuttall AL (1997) The mechanical waveform of the basilar membrane. I. Frequency modulations (“glides”) in impulse responses and cross-correlation functions. J Acoust Soc Am 101:3583–3592
Dong W, Olson ES (2006) Middle ear forward and reverse transmission in gerbil. J Neurophys 95:2951–2961
Ellison JC, Keefe DH (2005) Audiometric predictions using stimulus-frequency otoacoustic emissions and middle ear measurements. Ear Hear 26:487–503
Eustaquio-Martin A, Lopez-Poveda EA (2011) Isoresponse versus isoinput estimates of cochlear filter tuning. J Assoc Res Otolaryngol 12:281–299
Fallah E, Strimbu CE, Olson ES (2019) Nonlinearity and amplification in cochlear responses to single and multi-tone stimuli. Hear Res 377:271–281
Farina A (2000) Simultaneous measurement of impulse response and distortion with a swept-sine technique. In: 108th AES Convention. Paris
Goodman SS, Mertes IB, Scheperle RA (2011) Delays and growth rates of multiple TEOAE components. In: What fire is in mine ears: progress in auditory biomechanics (Shera CA, Olson ES, eds), pp 279–285. Williamstown, MA: Melville, New York
Goodman SS, Fitzpatrick DF, Ellison JC, Jesteadt W, Keefe DH (2009) High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans. J Acoust Soc Am 125:1014–1032
Guinan JJ (1990) Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats. In: Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR (eds) The mechanics and biophysics of hearing. Springer-Verlag, Madison, pp 170–177
Huang S, Olson ES (2011) Auditory nerve excitation via a non-traveling wave mode of basilar membrane motion. J Assoc Res Otolaryngol 12:559–575
Kalluri R, Shera CA (2007a) Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 121:2097–2110
Kalluri R, Shera CA (2007b) Comparing stimulus-frequency otoacoustic emissions measured by compression, suppression, and spectral smoothing. J Acoust Soc Am 122:3562–3575
Keefe DH (2012) Moments of click-evoked otoacoustic emissions in human ears: group delay and spread, instantaneous frequency and bandwidth. J Acoust Soc Am 132:3319–3350
Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DF (2016) Comparisons of transient evoked otoacoustic emissions using chirp and click stimuli. J Acoust Soc Am 140:1949–1973
Kemp DT, Chum RA (1980) Observations on the generator mechanism of stimulus frequency acoustic emissions–two tone suppression. In: deBoer E, Viergever MA (eds) Psychophysical, physiological and behavioral studies in hearing. Delft University Press, Delft, pp 34–41
Lewis JD, Goodman SS (2014) The effect of stimulus bandwidth on the nonlinear-derived tone-burst-evoked otoacoustic emission. J Assoc Res Otolaryngol 15:915–931
Lewis JD, Goodman SS (2015) Basal contributions to short-latency transient-evoked otoacoustic emission components. J Assoc Res Otolaryngol 16:29–45
Long GR, Talmadge CL, Lee J (2008) Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am 124:1613–1626
Moleti A, Sisto R (2020) Does the “reticular lamina nonlinearity” contribute to the basal DPOAE source? J Assoc Res Otolaryngol 21:463–473
Moleti A, Longo F, Sisto R (2012a) Time-frequency domain filtering of evoked otoacoustic emissions. J Acoust Soc Am 132:2455–2467
Moleti A, Botti T, Sisto R (2012b) Transient-evoked otoacoustic emission generators in a nonlinear cochlea. J Acoust Soc Am 131:2891–2903
Moleti A, Sisto R, Lucertini M (2014) Experimental evidence for the basal generation place of the short-latency transient-evoked otoacoustic emissions. J Acoust Soc Am 135:2862–2872
Moleti A, Al-Maamury AM, Bertaccini D, Botti T, Sisto R (2013) Generation place of the long- and short-latency components of transient-evoked otoacoustic emissions in a nonlinear cochlear model. J Acoust Soc Am 133:4098–4108
Muller M (1996) The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res 94:148–156
Novak A, Lotton P, Simon L (2015) Synchronized swept-sine: Theory, application, and implementation. J Audio Eng Soc 63:786–798
Novak A, Simon L, Kadlec F, Lotton P (2010) Nonlinear system identification using exponential swept-sine signal. IEEE Trans Instrum Meas 59:2220–2229
Ohlemiller KK, Echteler SM (1990) Functional correlates of characteristic frequency in single cochlear nerve fibers of the Mongolian gerbil. J Comp Physiol A 167:329–338
Rabiner LR, Schafer RW (2007) Introduction to digital speech processing: Now Publishers.
Recio-Spinoso A, Narayan SS, Ruggero MA (2009) Basilar membrane responses to noise at a basal site of the chinchilla cochlea: quasi-linear filtering. J Assoc Res Otolaryngol 10:471–484
Recio A, Rhode WS (2000) Basilar membrane responses to broadband stimuli. J Acoust Soc Am 108:2281–2298
Recio A, Rich NC, Narayan SS, Ruggero MA (1998) Basilar-membrane responses to clicks at the base of the chinchilla cochlea. J Acoust Soc Am 103:1972–1989
Ren T, Nuttall AL (2001) Basilar membrane vibration in the basal turn of the sensitive gerbil cochlea. Hear Res 151:48–60
Robles L, Ruggero MA (2001) Mechanics of the mammalian cochlea. Physiol Rev 81:1305–1352
Schairer KS, Ellison JC, Fitzpatrick D, Keefe DH (2006) Use of stimulus-frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears. J Acoust Soc Am 120:901–914
Schmiedt RA (1982) Boundaries of two-tone rate suppression of cochlear-nerve activity. Hear Res 7:335–351
Schmiedt RA (1989) Spontaneous rates, thresholds and tuning of auditory-nerve fibers in the gerbil: Comparisons to cat data. Hear Res 42:23–35
Shera CA, Zweig G (1993) Noninvasive measurement of the cochlear traveling-wave ratio. J Acoust Soc Am 93:3333–3352
Shera CA, Guinan JJ Jr (1999) Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J Acoust Soc Am 105:782–798
Shera CA, Guinan JJ Jr (2003) Stimulus-frequency-emission group delay: A test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am 113:2762–2772
Shera CA, Guinan JJ (2008) Mechanisms of mammalian otoacoustic emission. In: Active processes and otoacoustic emissions in hearing (Manley GA, Fay RR, Popper AN, eds), pp 305–342. New York, NY: Springer New York
Shera CA, Bergevin C (2012) Obtaining reliable phase-gradient delays from otoacoustic emission data. J Acoust Soc Am 132:927–943
Shera CA, Cooper NP (2013) Basilar-membrane interference patterns from multiple internal reflection of cochlear traveling waves. J Acoust Soc Am 133:2224–2239
Shera CA, Guinan JJ Jr, Oxenham AJ (2002) Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci USA 99:3318–3323
Shera CA, Tubis A, Talmadge CL (2008) Testing coherent reflection in chinchilla: Auditory-nerve responses predict stimulus-frequency emissions. J Acoust Soc Am 124:381–395
Shera CA, Guinan JJ Jr, Oxenham AJ (2010) Otoacoustic estimation of cochlear tuning: Validation in the chinchilla. J Assoc Res Otolaryngol 11:343–365
Siegel JH (2007) Calibration of otoacoustic emission probes. In: Robinette MS, Glattke TJ (eds) Otoacoustic emissions: clinical applications, Third, Edition. Thieme, New York, pp 403–429
Siegel JH, Charaziak K, Cheatham MA (2011) Transient‐ and tone‐evoked otoacoustic emissions in three species. In: What fire is in mine ears: progress in auditory biomechanics (Shera C, Olson E, eds), pp 307–314
Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero MA (2005) Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am 118:2434–2443
Sisto R, Sanjust F, Moleti A (2013) Input/output functions of different-latency components of transient-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 133:2240–2253
Sisto R, Moleti A, Shera CA (2015) On the spatial distribution of the reflection sources of different latency components of otoacoustic emissions. J Acoust Soc Am 137:768–776
Talmadge CL, Tubis A, Long GR, Piskorski P (1998) Modeling otoacoustic emission and hearing threshold fine structures. J Acoust Soc Am 104:1517–1543
Talmadge CL, Tubis A, Long GR, Tong C (2000) Modeling the combined effects of basilar membrane nonlinearity and roughness on stimulus frequency otoacoustic emission fine structure. J Acoust Soc Am 108:2911–2932
Tognola G, Grandori F, Ravazzani P (1997) Time-frequency distributions of click-evoked otoacoustic emissions. Hear Res 106:112–122
Vencovský V, Vetešník A, Gummer AW (2020) Nonlinear reflection as a cause of the short-latency component in stimulus-frequency otoacoustic emissions simulated by the methods of compression and suppression. J Acoust Soc Am 147:3992
Versteegh CPC, van der Heijden M (2012) Basilar membrane responses to tones and tone complexes: Nonlinear effects of stimulus intensity. J Assoc Res Otolaryngol 13:785–798
Withnell RH, Yates GK (1998) Enhancement of the transient-evoked otoacoustic emission produced by the addition of a pure tone in the guinea pig. J Acoust Soc Am 104:344–349
Withnell RH, McKinley S (2005) Delay dependence for the origin of the nonlinear derived transient evoked otoacoustic emission. J Acoust Soc Am 117:281–291
Yates GK, Withnell RH (1999) The role of intermodulation distortion in transient-evoked otoacoustic emissions. Hear Res 136:49–64
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98:2018–2047
Acknowledgements
We thank Alessandro Altoè for many helpful discussions.
Funding
This study was financially supported by grants K99 DC016906-01A1 (KKC) and R01 DC003687 (CAS) from the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Charaziak, K.K., Shera, C.A. Reflection-Source Emissions Evoked with Clicks and Frequency Sweeps: Comparisons Across Levels. JARO 22, 641–658 (2021). https://doi.org/10.1007/s10162-021-00813-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-021-00813-3