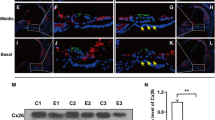
Abstract
Incomplete partition type II (IP-II) is frequently identified in ears with SLC26A4 mutations. Cochleae with IP-II are generally observed to have 1½ turns; the basal turns are normally formed, and the apical turn is dilated or cystic. The objective of this study was to characterize the pathomorphogenesis of the IP-II cochlear anomaly in Slc26a4-null mice. Otic capsules were dissected from Slc26a4Δ/+ and Slc26a4Δ/Δ mice at 1 and 8 days of age and at 1 and 3 months of age. X-ray micro-computed tomography was used to image samples. We used a multiplanar view and three-dimensional reconstructed models to calculate the cochlear duct length, cochlear turn rotation angle, and modiolus tilt angle. The number of inner hair cells was counted, and the length of the cochlear duct was measured in a whole-mount preparation of the membranous labyrinth. X-ray micro-computed tomography mid-modiolar planar views demonstrated cystic apical turns in Slc26a4Δ/Δ mice resulting from the loss or deossification of the interscalar septum, which morphologically resembles IP-II in humans. Planes vertical to the modiolus showed a similar mean rotation angle between Slc26a4Δ/+ and Slc26a4Δ/Δ mice. In contrast, the mean cochlear duct length and mean number of inner hair cells in Slc26a4Δ/Δ mice were significantly smaller than in Slc26a4Δ/+ mice. In addition, there were significant differences in the mean tilt angle and mean width of the modiolus. Our analysis of Slc26a4-null mice suggests that IP-II in humans reflects loss or deossification of the interscalar septum but not a decreased number of cochlear turns.







Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Al-Amri SS, Kalyankar NV, Khamitkar, SD (2010) Image segmentation by using threshold techniques arXiv preprint 1005:4020. https://arxiv.org/abs/1005.4020
Altmann F (1950) Histologic picture of inherited nerve deafness in man and animals. Arch Otolaryngol 51:852–890
Bajin MD, Pamuk AE, Pamuk G, Ozgen B, Sennaroglu L (2018) The association between modiolar base anomalies and intraoperative cerebrospinal fluid leakage in patients with incomplete partition type-II anomaly: a classification system and presentation of 73 cases. Otol Neurotol 39:e538-e542. https://doi.org/10.1097/MAO.0000000000001871
Chan BKC (2018) Data analysis using R programming. Adv Exp Med Biol 1082:47–122. https://doi.org/10.1007/978-3-319-93791-5_2
Chang YN Jaumann EA, Reichel K, Hartmann J, Oliver D, Hummer G, Joseph B, Geertsma ER. (2019) Structural basis for functional interactions in dimers of SLC26 transporters. Nat Commun 10:2032. https://doi.org/10.1038/s41467-019-10001-w
Choi BY, Kim HM, Ito T, Lee KY, Li X, Monahan K, Wen Y, Wilson E, Kurima K, Saunders TL, Petralia RS, Wangemann P, Friedman TB, Griffith AJ. (2011) Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. Journal Clin Invest 121:4516–4525. https://doi.org/10.1172/JCI59353
Coleman B, Rickard NA, de Silva MG, Shepherd RK (2009) A protocol for cryoembedding the adult guinea pig cochlea for fluorescence immunohistology. J Neurosci Methods 176:144–151. https://doi.org/10.1016/j.jneumeth.2008.09.007
Ding X, Tian H, Wang W, Zhang D (2009) Cochlear implantation in China: review of 1,237 cases with an emphasis on complications. ORL J Otorhinolaryngol Relat Spec 71:192–195. https://doi.org/10.1159/000229297
Dror AA, Politi Y, Shahin H, Lenz DR, Sossena S, Nofziger C, Fuchs H, de Angelis MH, Paulmichl M, Weiner S, Avraham KB. (2010) Calcium oxalate stone formation in the inner ear as a result of an Slc26a4 mutation. J Biol Chem 285:21724–21735. https://doi.org/10.1074/jbc.M110.120188
Elfarnawany M, Alam SR, Rohani S, Zhu N, Agrawal S, Ladak H (2017) Micro-CT versus synchrotron radiation phase contrast imaging of human cochlea. J Microsc 265:349–357
Everett LA, Belyantseva A, Knoben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. (2001) Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10:153–161. https://doi.org/10.1093/hmg/10.2.153
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Filion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Fujikawa T, Petralia RS, Fitzgerald TS, Wang Y-X, Millis B, Morgado-Diaz JA, Kitamura K, Kachar B. (2014) Localization of kainate receptors in inner and outer hair cell synapses. Hearing Res 314:20–32. https://doi.org/10.1016/j.heares.2014.05.001
Gussen R (1968) Mondini type of genetically determined deafness. J Laryngol Otol 82:41–55. https://doi.org/10.1017/s002221510006847x
Honda K, Noguchi Y, Kawashima Y, Takahashi M, Nishio A, Kitamura K (2015) Ex vivo visualization of the mouse otoconial layer compared with micro-computed tomography. Otol Neurotol 36:311–317. https://doi.org/10.1097/MAO.0000000000000376
Hongjian L, Guangke W, Song M, Xiaoli D, Daoxing Z (2012) The prediction of CSF gusher in cochlear implants with inner ear abnormality. Acta Otolaryngol 132:1271–1274. https://doi.org/10.3109/00016489.2012.701328
Hvidberg-Hansen J, Jorgensen MB (1968) The inner ear in Pendred’s syndrome. Acta Otolaryngol 66:129–135
Ito T, Choi BM, King KA, Zalewski CK, Muskett J, Chattaraj P, Shawker T, Reynolds JC, Butman JA, Brewer CC, Wangemann P, Alper SL, Griffith AJ. (2011) SLC26A4 genotypes and phenotypes associated with enlargement of the vestibular aqueduct. Cell Physiol Biochem 28:545–552. https://doi.org/10.1159/000335119
Ito T, Li X, Kurima K, Choi BY, Wangemann P, Griffith AJ (2014) Slc26a4-insufficiency causes fluctuating hearing loss and stria vascularis dysfunction. Neurobiology of disease 66C:53–65. https://doi.org/10.1016/j.nbd.2014.02.002
Jackler RK, Luxford WM, House WF (1987) Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 97:2–14. https://doi.org/10.1002/lary.5540971301
Johnsen T, Jorgensen MB, Johnsen S (1986) Mondini cochlea in Pendred’s syndrome. A Histological Study. Acta Otolaryngol 102:239–247
Jui-Cheng Y, Fu-Juay C, Shyang C (1995) A new criterion for automatic multilevel thresholding. IEEE Trans Image Process 4:370–378. https://doi.org/10.1109/83.366472
Kempf HG, Tempel S, Johann K, Lenarz T (1999) Complications of cochlear implant surgery in children and adults. Laryngorhinootologie 78:529–537. https://doi.org/10.1055/s-1999-8753
Kim BG, Sim NS, Kim SH, Kim UK, Kim S, Choi JY (2013) Enlarged cochlear aqueducts: a potential route for CSF gushers in patients with enlarged vestibular aqueducts. Otol Neurotol 34:1660–1665. https://doi.org/10.1097/MAO.0b013e3182a036e4
Kim HM, Wangemann P (2011) Epithelial Cell Stretching and Luminal Acidification Lead to a Retarded Development of Stria Vascularis and Deafness in Mice Lacking Pendrin. PloS One 6:e17949. https://doi.org/10.1371/journal.pone.0017949
Leung KJ, Quesnel AM, Juliano AF, Curtin HD (2016) Correlation of CT, MR, and histopathology in incomplete partition-II cochlear anomaly. Otol Neurotol 37:434–437. https://doi.org/10.1097/mao.0000000000001027
Makary C, Shin J, Caruso P, Curtin H, Merchant S (2010) A Histological Study of Scala Communis with Radiological Implications. Audiology & Neuro-Otology 15:383–393. https://doi.org/10.1159/000307345
Mey K, Bille M, Caye-Thomasen P (2016) Cochlear implantation in Pendred syndrome and non-syndromic enlarged vestibular aqueduct - clinical challenges, surgical results, and complications. Acta Otolaryngol 136:1064–1068. https://doi.org/10.1080/00016489.2016.1185538
Mondini C (1791) Anatomica surdi nadti sectio Die Bononiensi Scientiarunz et articltm instituto atque academic comnlentarii 7:p419–431
O'Malley Jr BW, Li D, Turner DS (1995) Hearing loss and cochlear abnormalities in the congenital hypothyroid (hyt/hyt) mouse. Hearing Res 88:181–189
Phelps PD (1990) Mondini and “pseudo Mondini.” Clin Otolaryngol Allied Sci 15:99–101
Roesch S, Bernardinelli E, Nofziger C, Toth M, Patsch W, Rasp G, Paulmichl M, Dossena S. (2018) Functional testing of SLC26A4 variants-clinical and molecular analysis of a cohort with enlarged vestibular aqueduct from Austria. Int J Mol Sci 19. https://doi.org/10.3390/ijms19010209
Roesch S, Moser G, Rasp G, Toth M (2013) CT-scans of cochlear implant patients with characteristics of Pendred syndrome. Cell Physiol Biochem 32:166–172. https://doi.org/10.1159/000356636
Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC, Green ED (2003) Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in pendred syndrome. J Assoc Res Otolaryngol 4:394–404. https://doi.org/10.1007/s10162-002-3052-4
Schuknecht HF, Merchant SN, Nadol JB (2010) Schuknecht's pathology of the ear. 3rd edn. People's Medical Pub. House-USA, Shelton, CT
Sennaroglu L (2016) Histopathology of inner ear malformations: do we have enough evidence to explain pathophysiology? Cochlear Implants Int 17:3–20. https://doi.org/10.1179/1754762815y.0000000016
Sennaroglu L, Saatci I (2002) A New Classification for Cochleovestibular Malformations. Laryngoscope 112:2230–2241. https://doi.org/10.1097/00005537-200212000-00019
Sennaroglu L, Saatci I (2004) Unpartitioned versus incompletely partitioned cochleae: radiologic differentiation. Otol Neurotol 25:520–529; discussion 529. https://doi.org/10.1097/00129492-200407000-00020
Sieber D, Erfurt P, John S, Santos GRD, Schurzig D, Sorensen MS, Lenarz T (2019) The OpenEar library of 3D models of the human temporal bone based on computed tomography and micro-slicing. Sci Data 6:180297. https://doi.org/10.1038/sdata.2018.297
Takeda H, Miwa T, Kim MY, Choi BY, Orita Y, Minoda R (2019) Prenatal electroporation-mediated gene transfer restores Slc26a4 knock-out mouse hearing and vestibular function. Scientific Reports 9. https://doi.org/10.1038/s41598-019-54262-3
Uziel A, Pujol R, Legrand C, Legrand J (1983) Cochlear synaptogenesis in the hypothyroid rat. Dev Brain Res 7:295–301
Velazquez ER, Parmar C, Jermoui M, Mak RH, van Baardwijk A, Fennessy FM, Lewis JH, De Ruysscher D, Kikinis R, Philippe L, Aerts HJWL. (2013) Volumetric CT-based segmentation of NSCLC using 3D-Slicer. Scientific Reports 3:3529. https://doi.org/10.1038/srep03529
Walter JD, Sawicka M, Dutzler R (2019) Cryo-EM structures and functional characterization of murine Slc26a9 reveal mechanism of uncoupled chloride transport. Elife 8. https://doi.org/10.7554/eLife.46986
Wangemann P, Kim HM, Billings S, Nakaya K, Li X, Singh R, Sharlin DS, Forrest D, Marcus DC, Fong P. (2009) Developmental delays consistent with cochlear hypothyroidism contribute to failure to develop hearing in mice lacking Slc26a4/pendrin expression. Am J Physiol Renal Physiol. 297:F1435–1447. https://doi.org/10.1152/ajprenal.00011.2009
Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. (2007) Loss of cochlear HCO3-secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol 292:F1345-1353. https://doi.org/10.1152/ajprenal.00487.2006
Wootten CT, Backous DD, Haynes DS (2006) Management of cerebrospinal fluid leakage from cochleostomy during cochlear implant surgery. Laryngoscope 116:2055–2059. https://doi.org/10.1097/01.mlg.0000240286.43289.87
Zhu L, Kolesov I, Gao Y, Kikinis R, Tannenbaum A An effective interactive medical image segmentation method using fast growcut. In: MICCAI workshop on interactive medical image computing, 2014.
Funding
Supported by a Grant-in-Aid for Scientific Research (grant No. 17K11316, 17K11314) from the Ministry of Health, Labor, and Welfare of Japan, NIDCD intramural research fund Z01-DC-000060, and a research grant from Kao Melanin Workshop.
Author information
Authors and Affiliations
Contributions
T.I. designed the research, performed the experiments, analyzed the data, and wrote the manuscript. T.F., K.H., and A.M. performed the experiments. H.W., J.B., Y. K., and T. M. provided critical feedback. A.G. and T.T. helped guide the research, analysis, and manuscript. All of the authors read and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University (No. A2019-280A).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ito, T., Fujikawa, T., Honda, K. et al. Cochlear Pathomorphogenesis of Incomplete Partition Type II in Slc26a4-Null Mice. JARO 22, 681–691 (2021). https://doi.org/10.1007/s10162-021-00812-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-021-00812-4