Abstract
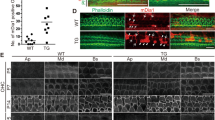
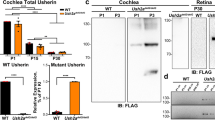
Tprn encodes the taperin protein, which is concentrated in the tapered region of hair cell stereocilia in the inner ear. In humans, TPRN mutations cause autosomal recessive nonsyndromic deafness (DFNB79) by an unknown mechanism. To determine the role of Tprn in hearing, we generated Tprn-null mice by clustered regularly interspaced short palindromic repeat/Cas9 genome-editing technology from a CBA/CaJ background. We observed significant hearing loss and progressive degeneration of stereocilia in the outer hair cells of Tprn-null mice starting from postnatal day 30. Transmission electron microscopy images of stereociliary bundles in the mutant mice showed some stereociliary rootlets with curved shafts. The central cores of the stereociliary rootlets possessed hollow structures with surrounding loose peripheral dense rings. Radixin, a protein expressed at stereocilia tapering, was abnormally dispersed along the stereocilia shafts in Tprn-null mice. The expression levels of radixin and β-actin significantly decreased.We propose that Tprn is critical to the retention of the integrity of the stereociliary rootlet. Loss of Tprn in Tprn-null mice caused the disruption of the stereociliary rootlet, which resulted in damage to stereociliary bundles and hearing impairments. The generated Tprn-null mice are ideal models of human hereditary deafness DFNB79.
Similar content being viewed by others
References
Holley MC. Keynote review: The auditory system, hearing loss and potential targets for drug development. Drug Discov Today 2005; 10 (19): 1269–1282
Corwin JT, Warchol ME. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci 1991; 14(1): 301–333
Tilney LG, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol 1983; 96(3): 807–821
Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high-and lowfrequency cells and evidence for a connection to the lateral membrane. J Neurosci 2008; 28(25): 6342–6353
Müller U, Barr-Gillespie PG. New treatment options for hearing loss. Nat Rev Drug Discov 2015; 14(5): 346–365
Fettiplace R, Kim KX. The physiology of mechanoelectrical transduction channels in hearing. Physiol Rev 2014; 94(3): 951–986
Gagnon LH, Longo-Guess CM, Berryman M, Shin JB, Saylor KW, Yu H, Gillespie PG, Johnson KR. The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci 2006; 26(40): 10188–10198
Goodyear R, Richardson G. Distribution of the 275 kD hair cell antigen and cell surface specialisations on auditory and vestibular hair bundles in the chicken inner ear. J Comp Neurol 1992; 325(2): 243–256
Anderson DW, Probst FJ, Belyantseva IA, Fridell RA, Beyer L, Martin DM, Wu D, Kachar B, Friedman TB, Raphael Y, Camper SA. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet 2000; 9(12): 1729–1738
Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol 1997; 137(6): 1287–1307
Pataky F, Pironkova R, Hudspeth AJ. Radixin is a constituent of stereocilia in hair cells. Proc Natl Acad Sci USA 2004; 101(8): 2601–2606
Bau S, Schracke N, Kränzle M, Wu H, Stähler PF, Hoheisel JD, Beier M, Summerer D. Targeted next-generation sequencing by specific capture of multiple genomic loci using low-volume microfluidic DNA arrays. Anal Bioanal Chem 2009; 393(1): 171–175
Li Y, Pohl E, Boulouiz R, Schraders M, Nürnberg G, Charif M, Admiraal RJ, von Ameln S, Baessmann I, Kandil M, Veltman JA, Nürnberg P, Kubisch C, Barakat A, Kremer H, Wollnik B. Mutations in TPRN cause a progressive form of autosomalrecessive nonsyndromic hearing loss. Am J Hum Genet 2010; 86 (3): 479–484
Rehman AU, Morell RJ, Belyantseva IA, Khan SY, Boger ET, Shahzad M, Ahmed ZM, Riazuddin S, Khan SN, Riazuddin S, Friedman TB. Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet 2010; 86(3): 378–388
Salles FT, Andrade LR, Tanda S, Grati M, Plona KL, Gagnon LH, Johnson KR, Kachar B, Berryman MA. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton (Hoboken) 2014; 71(1): 61–78
Zhao B, Wu Z, Müller U. Murine Fam65b forms ring-like structures at the base of stereocilia critical for mechanosensory hair cell function. eLife 2016; 5: e14222
Chen M, Wang Q, Zhu GH, Hu P, Zhou Y, Wang T, Lai RS, Xiao ZA, Xie DH. Progressive hearing loss and degeneration of hair cell stereocilia in taperin gene knockout mice. Biochem Biophys Res Commun 2016; 479(4): 703–707
Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 1999; 130(1-2): 94–107
Fujihara Y, Ikawa M. CRISPR/Cas9-based genome editing in mice by single plasmid injection. Methods Enzymol 2014; 546: 319–336
Steigelman KA, Lelli A, Wu X, Gao J, Lin S, Piontek K, Wodarczyk C, Boletta A, Kim H, Qian F, Germino G, Géléoc GS, Holt JR, Zuo J. Polycystin-1 is required for stereocilia structure but not for mechanotransduction in inner ear hair cells. J Neurosci 2011; 31 (34): 12241–12250
Men Y, Zhang A, Li H, Zhang T, Jin Y, Li H, Zhang J, Gao J. LKB1 is required for the development and maintenance of stereocilia in inner ear hair cells in mice. PLoS One 2015; 10(8): e0135841
Lelli A, Asai Y, Forge A, Holt JR, Géléoc GS. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 2009; 101(6): 2961–2973
Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol 2005; 485(1): 75–85
Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, Ito J, Tsukita S, Tsukita S. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol 2004; 166(4): 559–570
Kolb CA, Käser MA, Kopecký J, Zotz G, Riederer M, Pfündel EE. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol 2001; 127(3): 863–875
Kopecky D, Hayde M, Prusa AR, Adlassnig KP. Knowledge-based interpretation of toxoplasmosis serology test results including fuzzy temporal concepts—the ToxoNet system. Stud Health Technol Inform 2001; 84(Pt 1): 484–488
Francis SP, Krey JF, Krystofiak ES, Cui R, Nanda S, Xu W, Kachar B, Barr-Gillespie PG, Shin JB. A short splice form of Xin-actin binding repeat containing 2 (XIRP2) lacking the Xin repeats is required for maintenance of stereocilia morphology and hearing function. J Neurosci 2015; 35(5): 1999–2014
Perrin BJ, Strandjord DM, Narayanan P, Henderson DM, Johnson KR, Ervasti JM. β-Actin and fascin-2 cooperate to maintain stereocilia length. J Neurosci 2013; 33(19): 8114–8121
Khan SY, Riazuddin S, Shahzad M, Ahmed N, Zafar AU, Rehman AU, Morell RJ, Griffith AJ, Ahmed ZM, Riazuddin S, Friedman TB. DFNB79: reincarnation of a nonsyndromic deafness locus on chromosome 9q34.3. Eur J Hum Genet 2010; 18(1): 125–129
Zheng L, Sekerková G, Vranich K, Tilney LG, Mugnaini E, Bartles JR. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 2000; 102(3): 377–385
Peng AW, Belyantseva IA, Hsu PD, Friedman TB, Heller S. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J Neurosci 2009; 29(48): 15083–15088
Sekerková G, Richter CP, Bartles JR. Roles of the espin actinbundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet 2011; 7(3): e1002032
Furness DN, Johnson SL, Manor U, Rüttiger L, Tocchetti A, Offenhauser N, Olt J, Goodyear RJ, Vijayakumar S, Dai Y, Hackney CM, Franz C, Di Fiore PP, Masetto S, Jones SM, Knipper M, Holley MC, Richardson GP, Kachar B, Marcotti W. Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc Natl Acad Sci USA 2013; 110(34): 13898–13903
Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat Rev Genet 2004; 5(7): 489–498
Petit C, Richardson GP. Linking genes underlying deafness to hairbundle development and function. Nat Neurosci 2009; 12(6): 703–710
Drummond MC, Belyantseva IA, Friderici KH, Friedman TB. Actin in hair cells and hearing loss. Hear Res 2012; 288(1-2): 89–99
Shahin H, Walsh T, Sobe T, Abu Sa’ed J, Abu Rayan A, Lynch ED, Lee MK, Avraham KB, King MC, Kanaan M. Mutations in a novel isoform of TRIOBP that encodes a filamentous-actin binding protein are responsible for DFNB28 recessive nonsyndromic hearing loss. Am J Hum Genet 2006; 78(1): 144–152
Tilney LG, Jaffe LA. Actin, microvilli, and the fertilization cone of sea urchin eggs. J Cell Biol 1980; 87(3): 771–782
Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA 3rd, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 2010; 141(5): 786–798
Boutet de Monvel J, Petit C. Wrapping up stereocilia rootlets. Cell 2010; 141(5): 748–750
Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003; 112(4): 453–465
Zheng QY, Tong YC, Alagramam KN, Yu H. Tympanometry assessment of 61 inbred strains of mice. Hear Res 2007; 231(1-2): 23–31
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 Program, No. 2014CB541703), the National Natural Science Foundation of China (No. 81670943), and the Natural Science Foundation of Shandong Province (No. ZR2015PC020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Yuqin Men, Xiujuan Li, Hailong Tu, Aizhen Zhang, Xiaolong Fu, Zhishuo Wang, Yecheng Jin, Congzhe Hou, Tingting Zhang, Sen Zhang, Yichen Zhou, Boqin Li, Jianfeng Li, Xiaoyang Sun, Haibo Wang, and Jiangang Gao declare that they have no conflict of interest. All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Men, Y., Li, X., Tu, H. et al. Tprn is essential for the integrity of stereociliary rootlet in cochlear hair cells in mice. Front. Med. 13, 690–704 (2019). https://doi.org/10.1007/s11684-018-0638-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-018-0638-8