Abstract
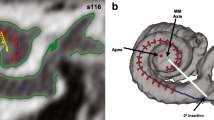
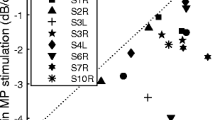
Variability in speech perception scores among cochlear implant listeners may largely reflect the variable efficacy of implant electrodes to convey stimulus information to the auditory nerve. In the present study, three metrics were applied to assess the quality of the electrode-neuron interface of individual cochlear implant channels: the electrically evoked compound action potential (ECAP), the estimation of electrode position using computerized tomography (CT), and behavioral thresholds using focused stimulation. The primary motivation of this approach is to evaluate the ECAP as a site-specific measure of the electrode-neuron interface in the context of two peripheral factors that likely contribute to degraded perception: large electrode-to-modiolus distance and reduced neural density. Ten unilaterally implanted adults with Advanced Bionics HiRes90k devices participated. ECAPs were elicited with monopolar stimulation within a forward-masking paradigm to construct channel interaction functions (CIF), behavioral thresholds were obtained with quadrupolar (sQP) stimulation, and data from imaging provided estimates of electrode-to-modiolus distance and scalar location (scala tympani (ST), intermediate, or scala vestibuli (SV)) for each electrode. The width of the ECAP CIF was positively correlated with electrode-to-modiolus distance; both of these measures were also influenced by scalar position. The ECAP peak amplitude was negatively correlated with behavioral thresholds. Moreover, subjects with low behavioral thresholds and large ECAP amplitudes, averaged across electrodes, tended to have higher speech perception scores. These results suggest a potential clinical role for the ECAP in the objective assessment of individual cochlear implant channels, with the potential to improve speech perception outcomes.






Similar content being viewed by others
References
Abbas PJ, Brown CJ, Shallop JK, Firszt JB, Hughes ML, Hong SH, Staller SJ (1999) Summary of results using the nucleus CI24M implant to record the electrically evoked compound action potential. Ear Hear 20:45–59
Abbas PJ, Hughes ML, Brown CJ, Miller CA, South H (2004) Channel interaction in cochlear implant users evaluated using the electrically evoked compound action potential. Audiol Neurotol 9:203–213
Aschendorff A, Kromeier J, Klenzner T, Laszig R (2007) Quality control after insertion of the Nucleus Contour and Contour Advance electrode in adults. Ear Hear 28:75S–79S
Bierer JA (2007) Threshold and channel interaction in cochlear implant users: evaluation of the tripolar electrode configuration. J Acoust Soc Am 121:1642–1653
Bierer JA (2010) Probing the electrode-neuron interface with focused electrode stimulation. Trends Amplif 4(2):84–95
Bierer JA, Bierer KHA, Oxenham AJ (2015) A Fast Method for Measuring Psychophysical Thresholds across the Cochlear Implant Array. Trends Hear 19:1–12
Bierer JA, Faulkner KF (2010) Identifying low-functioning cochlear implant channels based on partial tripolar, single-channel thresholds and psychophysical tuning curves. Ear Hear 2:247–258
Bierer JA, Faulkner KF, Tremblay KL (2011) Identifying cochlear implant channels with poor electrode-neuron interface: electrically-evoked auditory brainstem responses measured with the partial-tripolar configuration. Ear Hear 32(4):436–444
Bierer JA, Nye AD (2014) Comparisons between detection threshold and loudness perception for individual cochlear implant channels. Ear Hear 35:641–651
Boëx C, Kos MI, Pelizzone M (2003) Forwarding masking in different cochlear implant systems. J Acoust Soc Am 114:2058–2065
Briaire JJ, Frijns JHM (2005) Unraveling the electrically evoked compound action potential. Hear Res 205:143–156
Brown CJ, Abbas PJ, Gantz B (1990) Electrically evoked whole-nerve action potentials: data from human cochlear implant users. J Acoust Soc Am 88(3):1385–1391
Cohen LT, Richardson LM, Saunders E, Cowan RSC (2003) Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking. Hear Res 179:72–87
Cohen LT, Saunders E, Richardson LM (2004) Spatial spread of neural excitation: comparison of compound action potential and forward-masking data in cochlear implant recipients. Int J Audiol 43:346–355
Crew JD, Galvin JJ, Fu QJ (2012) Channel interaction limits melodic pitch perception in stimulated cochlear implants. J Acoust Soc Am 132:429–435
Dillier N, Lai WK, Almqvist B, Frohne C, Muller-Deile J, Stecker M, Von Wallenberg E (2002) Measurement of the electrically evoked compound action potential via a neural response telemetry system. Ann Otol Rhinol Laryngol 111:407–414
Finley CC, Holden TA, Holden LK, Whiting BR, Chole RA, Neely GJ, Hullar TE, Skinner MW (2008) Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 29:920–928
Goldwyn JH, Bierer SM, Bierer JA (2010) Modeling the electrode-neuron interface of cochlear implants: effects of neural survival, electrode placement, and the partial tripolar configuration. Hear Res 268:93–104
Grolman W, Maat A, Verdam F, Simis Y, Carelsen B, Freling N, Tange R (2009) Spread of excitation measurements for the detection of electrode array foldovers: a prospective study comparing 3-dimensional rotational x-ray and intraoperative spread of excitation measurements. Otol Neurotol 30:27–33
Hall RD (1990) Estimation of surviving ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hear Res 49:155–168
Holden LK, Finley CC, Firszt JB, Holden TA, Brenner C, Potts LG, Gotter BD, Vanderhoof SS, Mispagel K, Hydebrand G, Skinner MW (2013) Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 34:342–360
Hughes ML (2008) A re-evaluation of the relation between physiological channel interaction and electrode pitch ranking in cochlear implants. Acoust Soc Am 124(5):2711–2714
Hughes ML (2013) Electrically evoked compound action potential. In: Zwolan T, Wolfe J (eds) Objective Measures in Cochlear Implants. Plural Publishing, Inc, San Diego
Hughes ML, Abbas PJ (2006a) The relation between electrophysiologic channel interaction and pitch ranking in cochlear implant recipients. Acoust Soc Am 119(3):1527–1537
Hughes ML, Abbas PJ (2006b) Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus perimodiolar cochlear implant electrode arrays. Acoust Soc Am 119(3):1538–1547
Hughes ML, Stille LJ (2008) Psychophysical versus physiological spatial forward masking and the relation to speech perception in cochlear implants. Ear Hear 29:435–452
Hughes ML, Stille LJ (2010) Effect of stimulus and recording parameters on spatial spread of excitation and masking patterns obtained with the electrically evoked compound action potential in cochlear implants. Ear Hear 31:679–692
Jolly CN, Spelman FA, Clopton BM (1996) Quadrupolar stimulation of cochlear prostheses: modeling and experimental data. IEEE Trans Biomed Eng 43(8):857–865
Jeon EK, Brown CJ, Etler CP, O’Brien S, Chiou L, Abbas PJ (2010) Comparison of Electrically Evoked Compound Action Potential Thresholds and Loudness Estimates for the Stimuli Used to Program the Advanced Bionics Cochlear Implant. J Am Acad Audiol 21(1):16–27
Jones GL, Won JH, Drennan WR, Rubinstein JT (2013) Relationship between channel interaction and spectral-ripple discrimination in cochlear implant users. J Acoust Soc Am 133(1):425–433
Kalkman RK, Briaire JJ, Frijns JHM (2015) Current focussing in cochlear implants: an analysis of neural recruitment in a computational model. Hear Res 322:89–98
Kim JR, Abbas PJ, Brown CJ, Elter CP, O’Brien S, Kim L (2010) The relationship between electrically evoked compound action potential and speech perception: a study in cochlear implant users with short electrode array. Otol Neurotol 31:1041–1048
Lai WK, Dillier N (2000) A simple two-component model of the electrically evoked compound action potential in the human cochlea. Audio Neurotol 5:333–345
Landsberger DM, Padilla M, Srinivasan AG (2012) Reducing current spread using current focusing in cochlear implant users. Hear Res 284:16–24
Landsberger DM, Srinivasan AG (2009) Virtual channel discrimination is improved by current focusing in cochlear implant recipients. Hear Res 254:34–41
Levitt H (1971) Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49:467–477
Litvak LM, Spahr AJ, Emadi G (2007) Loudness growth observed under partially tripolar stimulation: model and data from cochlear implant listeners. J Acoust Soc Am 122:967–981
Long CJ, Holden TA, McClelland GH, Parkinson WS, Shelton C, Kelsall DC, Smith ZM (2014) Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolayngol 15:293–304
Mens LHM (2007) Advances in cochlear implant telemetry: evoked neural responses, electrical field imaging, and technical integrity. Trends Amplif 11:143–159
Miller CA, Abbas PJ, Robinson BK (1994) The use of long-duration current pulses to assess neural survival. Hear Res 78:11–26
Miller CA, Brown CJ, Abbas PJ, Sui-Ling C (2008) The clinical application of potentials evoked from the peripheral auditory system. Hear Res 242:184–197
Nelson DA, Donaldson GS, Kreft H (2008) Forward-masked spatial tuning curves in cochlear implant users. J Acoust Soc Am 123:1522–1543
Noble JH, Labadie RF, Gifford RH, Dawant BM (2013) Image guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE 21:820–829
Pfingst BE, Xu L (2004) Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolayngol 5:11–24
Pfingst BE, Bowling SA, Colesa DJ, Garadat SN, Raphael Y, Shibata SB, Zhou N (2011) Cochlear infrastructure for electrical hearing. Hear Res 281:65–73
Pfingst BE, Xu L, Thompson CS (2004) Across-Site threshold variation in cochlear implants: relation to speech recognition. Audiolo Neurotol 9:341–352
Prado-Guitierrez P, Fewster LM, Heasman JM, McKay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:47–55
Ramekers D, Versnel H, Strahl SB, Smeets EM, Klis SFL, Grolman W (2014) Auditory-nerve responses to varied inter-phase gap and phase duration of the electric pulse stimulus as predictors for neuronal degeneration. J Assoc Res Otolaryngol 15:187–202
Scheperle RA, Abbas PJ (2015) Relationships Among Peripheral and Central Electrophysiological Measures of Spatial and Spectral Selectivity and Speech Perception in Cochlear Implant Users. Ear Hear. doi:10.1097/AUD.0000000000000144
Shepherd RK, Javel E (1997) Electrical stimulation of the auditory nerve I: correlation of physiological responses with cochlear status. Hear Res 108:112–144
Skinner MW, Holden TA, Whiting BR, Voie AH, Brunsden B, Neely JG, Saxon EA, Hullar TE, Finley CC (2007) In vivo estimates of the position of Advanced Bionics electrode arrays in the human cochlea. Ann Oto Rhino Laryngol 116:1–24
Smith L, Simmons FB (1983) Estimating eighth nerve survival by electrical stimulation. Ann Otol Rhinol Laryngol 92:19–23
Snel-Bongers J, Briaire JJ, Vanpoucke FJ, Frijns JHM (2012) Spread of excitation and channel interaction in single and dual-electrode cochlear implant stimulation. Ear Hear 33:367–376
Srinivasan AG, Landsberger DM, Shannon RV (2010) Current focusing sharpens local peaks of excitation in cochlear implant stimulation. Hear Res 270:89–100
Teymouri J, Hullar TE, Holden TA, Chole RA (2011) Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otol Neurotol 32:980–986
van der Marel KS, Briaire JJ, Verbist BM, Murrling TJ, Frijns JHM (2015) The influence of cochlear implant electrode position on performance. Audiol Neurol 20:202–211
Verbist BM, Frijns JHM, Geleijns J, van Buchem MA (2005) Multisection CT as a valuable tool in the postoperative assessment of cochlear implant patients. Am J Neuroradiol 26:424–429
Won JH, Drennan WR, Rubenstein JT (2007) Spectral-ripple discrimination correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolayngol 8:384–392
Acknowledgments
The authors would like to acknowledge Emily Ellis for assistance with data collection, Timothy Holden for analyzing the CT scans, and our subjects for their constant dedication. We also want to thank two anonymous reviewers for their insightful comments, Steven Bierer for his helpful editorial comments when drafting the manuscript, and Lynne A. Werner and the Communication Studies Participant Pool (P30 DC04661) for their support. Finally, we would also like to acknowledge our funding sources, RO1 DC012142 (JAB) and T32 DC 000033 (University of Washington Speech and Hearing Sciences: LAD, Boys Town National Research Hospital: RAS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
DeVries, L., Scheperle, R. & Bierer, J.A. Assessing the Electrode-Neuron Interface with the Electrically Evoked Compound Action Potential, Electrode Position, and Behavioral Thresholds. JARO 17, 237–252 (2016). https://doi.org/10.1007/s10162-016-0557-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-016-0557-9