Abstract
In cochlear implants, variation across stimulation sites in psychophysical detection thresholds (T levels) and maximum comfortable loudness levels (C levels) can be large when narrow-bipolar (BP) stimulation is used. This across-site variation is typically smaller when monopolar (MP) stimulation is used. At least two models can account for across-site variation and the effects of electrode configuration on the magnitude of the variation. According to one model, across-site variation reflects site-to-site differences in the distances between the stimulating electrodes and the sites of action-potential initiation. Under this model, the lower across-site variation with MP stimulation is due to shallower current versus distance gradients. An alternative model assumes that T and C levels depend on integration of activity across the whole population of neurons and that MP stimulation activates neurons over a larger spatial extent than does BP stimulation. If T and C levels are determined by integration of activity across large overlapping populations of neurons, then their values at adjacent sites should be more similar than if these levels result from integration across smaller, more independent populations. We tested the models by examining the effects on across-site variation of three variables believed to affect the spatial extent of activation: electrode configuration, stimulus level within the dynamic range, and electrode-array design. T levels and C levels were measured in 13 subjects with Nucleus® CI24M (straight array) and 9 subjects with Nucleus® CI24R(CS) (Contour) cochlear implants using bipolar (BP) and monopolar (MP) electrode configurations. Site-to-site variation in T and C levels for BP stimulation was 2.1–3.3 times larger than that for MP stimulation. Contrary to the across-neuron integration hypothesis, no significant differences were found between across-site variation for T levels and that for C levels for the BP configuration. There was considerable overlap in site-to-site variation values for the two types of implants but mean site-to-site variation in C levels for CI24M implants was significantly lower than that for CI24R(CS) implants. Control studies suggested that these results were not an artifact of the scale, and not due to differences in inherent variability of the psychophysical measures, or to the method of quantifying across-site variation.
Similar content being viewed by others
INTRODUCTION
A principal goal of current auditory-prosthesis research is to increase the number of functional channels available to the listener. With current technology, implanted subjects seem to have only about 8 functional channels, even when the number of electrodes and processor channels is much larger (Fishman et al. 1997; Fu et al. 1998; Friesen et al. 2001). Studies using acoustic simulations of auditory prostheses have shown that under difficult listening conditions or with challenging stimuli, subjects need more than 8 channels of information (Shannon et al. 1995; Dorman et al. 1998; Friesen et al. 2001; Xu et al. 2002). Several strategies have been proposed to increase the number of functional channels. One hypothesis is that if electrodes can be placed near the excitable neural elements, there will be less overlap in adjacent excited neural populations and more functional channels can be achieved.
For stimulating electrode arrays in the scala tympani, several factors probably contribute to the variation in length of current paths between the electrodes and the neurons. These include the medial–lateral position of the electrodes in the scala tympani (Kawano et al. 1998; Saunders et al. 2002), variation in the pattern of survival of neurons near the electrode array (Hinojosa and Marion 1983; Nadol 1997), and growth of tissue, particularly new dense bone, in the implanted cochlea. Considerable effort is being directed toward increasing the proximity of electrodes to neural tissue, including engineering implants that position electrodes close to the modiolar wall and tissue engineering to increase nerve survival in the implanted cochlea and/or to grow new neural tissue in close proximity to the implant. A noninvasive measure of the functional distance from the electrodes to the sites of action-potential initiation is needed in order to assess the success and/or functional benefits of these efforts. This study examines one potential measure: detection thresholds for narrow-bipolar stimulation.
In previous studies, we along with others have found large variation in psychophysical detection thresholds among nearby stimulation sites in the electrode array when narrow-bipolar stimulation is used (Busby et al. 1994; von Wallenberg et al. 1994; Pfingst et al. 1997). We suggest that this variation in thresholds is due in part to variation in the distance between the stimulation sites and the sites of neural activation. Since current level decreases as a function of distance from the electrodes, more current is required to activate neurons that are more distant from the electrodes. A second observation from previous studies is that the across-site variation in psychophysical detection thresholds for monopolar or broad-bipolar stimulation is lower than that for narrow-bipolar stimulation. These data are consistent with the threshold versus distance model because with monopolar stimulation, the activating current decreases less rapidly as a function of distance from the electrodes than is the case for narrow-bipolar stimulation (Kral et al. 1998).
However, another mechanism might also account for the smaller across-site variation seen with monopolar stimulation compared with narrow-bipolar stimulation. With monopolar stimulation, the shallower rate of current dissipation as a function of distance from the electrodes results in a broader activation pattern than when narrow-bipolar stimulation is used. Broader activation for monopolar stimulation has been confirmed by neurophysiological data (Hartmann and Klinke 1990; Bierer and Middlebrooks 2002). If detection thresholds (T levels) or maximum comfortable loudness levels (C levels) depends on integration of activity across the population of activated neurons, then one would expect electrode configuration to affect across-site variation in those levels. Specifically, the hypothesis is that when narrowly spaced BP stimulation restricts the spatial extent of neural activation to a small population of neurons, across-site variation can be large when there is local variation in the sensitivity of these small populations. In contrast, with MP stimulation, this hypothesis suggests that thresholds for nearby sites of stimulation depend on integration across large overlapping populations of neurons which reduces sensitivity to local variation in small populations of neurons. If this hypothesis is correct, then we would expect maximum comfortable loudness levels for narrow-bipolar stimulation to be much less variable from site to site compared with detection thresholds for the same configuration, because current spread and overlap of nearby stimulation sites increase as a function of stimulus level (Pfingst et al. 1985; Snyder et al. 1990; Kral et al. 1998; Bierer and Middlebrooks 2002). To test this hypothesis, in this study we compared across-site variation in C levels with that for T levels obtained from the same subjects.
In addition, we performed these experiments using two types of implants manufactured by Nucleus Ltd. (Lane Cove, Australia): the CI24M and the CI24R(CS). In the CI24M implant, the stimulating electrodes consist of platinum bands that encircle a silicone-rubber carrier. Prior to implantation this implant is straight; when it is inserted into the curved scala tympani, it tends to lie near the lateral wall (Shepherd et al. 1985; Kennedy 1987; Saunders et al. 2002). In contrast, the CI24R(CS) (Contour) implant has the electrodes positioned on only one side of the silicone-rubber carrier and the carrier is precurved and designed with the intention that, after insertion, the electrodes will lie close to the modiolar wall of the scala tympani (Cohen et al. 2002; Parkinson et al. 2002). Because of the intended proximity to the modiolus and having the electrodes on only one side of the carrier, this implant is assumed to require lower currents to activate neurons and reduce channel overlap. This is a relatively new strategy and its functional characteristics have not been studied extensively.
Assuming that electrode configuration, implant design, and/or stimulus level affect across-site variability, it is important to know whether these variables affect T and C level variability in general, or whether the effects are specific to variation across stimulation sites. To test this, we compared variation across stimulation sites to variation over repeated tests as a function of electrode configuration and level on a within-subjects basis. In addition, we tested detection thresholds using two psychophysical methods, including the relatively criterion-free two-interval forced-choice method to determine if a subject’s response criterion might contribute to across-site variation.
METHODS
Subjects
Twenty-two postlingually deaf subjects participated in the study. Thirteen had the Nucleus® CI24M implant and nine had the Nucleus® CI24R(CS) (Contour) implant. Subjects ranged from 29 to 78 years of age. They all received cochlear implants in the Department of Otolaryngology at the University of Michigan Health System. All subjects had at least six months of experience with the implants and were followed at the Cochlear Implant Program at the University of Michigan. Details for each subject are given in Table 1 with summary statistics in Table 2. Preimplant characteristics (age, duration of deafness, preoperative acoustic thresholds, etc.) did not differ appreciably between the groups of subjects with the two implant types. The use of human subjects in this research was reviewed and approved by the University of Michigan Medical School Institutional Review Board.
Research design
Independent variables
The independent variables in this study were (1) electrode configuration, (2) implant design, (3) stimulus level, and (4) psychophysical procedure.
Each subject was tested on two electrode configurations: BP and MP. In the BP configuration, stimulation was between two electrodes located next to each other in the electrode array. The spacing of the electrodes varied depending on the implant design. For CI24M implant the spacing of all adjacent electrodes was approximately 0.75 mm center to center. For the CI24R(CS) implant, the center-to-center spacing ranged from about 0.8 mm at the basal end of the implant to about 0.6 mm at the apical end, averaging about 0.64 mm. The MP configuration was a monopolar configuration (MP2 or MP1+2) in which the intracochlear electrode was labeled the “active” electrode. MP2 used a plate electrode located on the casing of the implanted receiver/stimulator as the return electrode, and MP1+2 used both the plate electrode and a ball electrode buried underneath the temporalis muscle as the return electrodes. For this study we treated MP2 and MP1+2 as equivalent and used the label “MP” for both.
Two implant designs were used: CI24M (13 subjects) and CI24R(CS) (9 subjects). As noted above, these implants differed in the geometry (shape, location, and spacing) of the electrodes and in the shape of the Silastic rubber carrier. The CI24M implant used band electrodes that surrounded the Silastic carrier and the array was straight prior to insertion into the scala tympani. The CI24R(CS) implant used half bands located on only one side of the Silastic carrier and the implant was precurved to match the medial aspect of the scala tympani with the intention that, when inserted into the cochlea, it would position the electrodes near the modiolar wall.
Testing was performed at two levels within the dynamic range. At the low level we measured psychophysical detection thresholds (T levels and TFC levels, as described below). At the high level we measured C levels.
To assess effects of psychophysical procedure, two procedures were used to estimate psychophysical detection thresholds: a method of adjustment and a two-interval forced choice (2IFC) method, as described in the Psychophysical Procedures subsection below. For this study, thresholds measured with the method of adjustment are called T levels and those measured with the 2IFC procedures are called TFC levels.
Dependent variables
The dependent variables in this study were across-site variation and test–retest variation in C levels and in T levels. For across-site variation, data were collected from all available sites in the 22-electrode arrays in the 22 subjects. “Stimulation site” was defined as the physical location of the electrodes through which current was passed to stimulate neurons. For MP stimulation there were 22 possible sites and for BP stimulation there were 21. A few sites in a few subjects were not tested because the sites were not functional or because stimulation at those sites produced uncomfortable sensations. Stimulation sites were labeled using the number of the “active” electrode. By convention, the more basal electrode in a bipolar pair was labeled as the “active” electrode and the more apical electrode was labeled the “return” electrode. For MP configurations, the scalar electrode was labeled “active.” The electrodes were numbered from 1 to 22 with electrode 1 being the most basal and 22 being the most apical electrode in the array. Thus, for example, for BP stimulation using electrodes 4 and 5, the stimulation site would be labeled site 4. The corresponding stimulation site for MP stimulation would be scalar electrode 4 referenced to an extracochlear return.
Three metrics were used to assess across-site variation. We defined “site-to-site variation” within subjects as the average absolute difference between adjacent electrodes in the level of current required to reach a given perception (detection threshold or maximum comfortable loudness). This was calculated by determining the differences in T levels, TFC levels, or C levels for each electrode in the array and its adjacent neighbor and then averaging the absolute values of all of these differences. Comparisons were included only if psychophysical values were obtained at two adjacent sites. Usually data were obtained for 20 adjacent sites in the BP condition and 21 adjacent sites in the MP condition. Ranges and standard deviations of all measured T, TFC, and C levels in the array were also calculated.
To test for inherent differences in variability of data collected under various conditions, we calculated standard deviations across repeated measures under fixed stimulus conditions (test–retest variability). For each of two independent variables, electrode configuration and stimulus level, standard deviations of 10 repeated measurements at each of 4 different sites were determined, and the mean test–retest standard deviation for the four sites was compared with standard deviation for one measurement each across 10 sites (across-site variability).
Test sessions
The experiment was divided into three sessions. Session 1 was always run first and all subjects participated in this session. Sessions 2 and 3 were run only for some of the subjects (as detailed in Table 1) and the order in which these sessions were run depended on the individual subject’s schedule.
In Session 1, the subjects’ T levels and C levels were measured for all available stimulation sites using the method of adjustment. The order of testing the two electrode configurations (BP or MP) was randomized. All 22 subjects participated in this session of the experiment.
In Session 2, 10 nonadjacent stimulation sites (i.e., the odd numbered sites 1 through 19, or the even-numbered sites 2 through 20) were selected to repeat the measurements of T and C levels for both BP and MP configurations. From these 10 sites, 4 were selected for 10 threshold measurements each. The 4 sites were selected to include those with high, low, and intermediate T and C levels. Thresholds for the remaining 6 sites were measured only once. The order of a total of 184 measurements (46 measurements of T levels plus 46 measurements of C levels times 2 configurations) was randomized. Thirteen of the 22 subjects participated in this session of the experiment (see Table 1).
In Session 3, TFC levels were measured at all available stimulation sites using the 2IFC method with flanking cues and adaptive tracking. In the beginning, the subjects practiced once or twice with the procedure. Feedback was provided during practice. No feedback was provided during the subsequent TFC level measurements. The measurements started with the most apical site and advanced to the most basal site. Thirteen of the 22 subjects participated in this session of the experiment (Table 1).
Hardware and software for electrical stimulation
A single, laboratory owned, SPrint® processor (Cochlear Corporation, Englewood, CO) was used for all subjects to assure uniformity in the external hardware. The measurements of T levels and C levels, using the method of adjustment, were controlled by the Cochlear Corporation’s Diagnostic and Programming System (DPS) software: Windows version R116 running on an IBM-compatible personal computer (PC). The measurements of detection threshold levels using the two-interval forced-choice method (TFC) were controlled by custom software run on the PC. This software generated sequences of frames and sent instructions to the SPrint processor utilizing Nucleus Implant Communicator® (version 3.27) software libraries. The custom software also provided a user interface that was used to collect the psychophysical responses from the subjects, as detailed below.
In both cases, the software communicated with the SPrint processor using an IF5 ISA card and a Processor Control Interface (PCI) (Cochlear Corporation). The SPrint processor controlled transmission of radio frequency (RF) pulses to the subject’s implanted receiver/stimulator. The implanted receiver/stimulator then decoded the RF information to the correct stimulation pulse parameters and delivered appropriate current pulses to the implanted cochlear electrode array.
Stimuli
Stimuli consisted of 200 µs/phase (in 20 subjects) or 300 µs/phase (in 2 subjects, S9 and S11) symmetric-biphasic pulses presented at a rate of 250 pulses/s. The longer pulse durations were required for two of the subjects in order to reach the C level within the current-output limits of the implanted receiver stimulators. For purposes of this experiment we treated these two pulse durations as equivalent. The polarity of the initial phase of each pulse to the “active” electrode was negative. The stimulus burst duration was 500 ms.
The implanted receiver-stimulators delivered current in 256 steps, specified in current level units (CL). Currents ranged from approximately 10 µApeak at CL = 0 to approximately 1750 µApeak at CL = 255 with a step size of approximately 2.046% (0.176 dB).
Output of the implanted receiver-stimulators at a given CL varied from stimulator to stimulator over a range of about ±10%. A calibration of the individual receiver-stimulators for each of the subjects was obtained from Cochlear Corporation. These calibrations gave the maximum output of the stimulator (I max; i.e., the output in microamps peak at CL = 255). The stimulator output in microamps peak [I(µApeak)] at any given CL was derived using the formula:

(Chris van den Honert, Cochlear Corporation, personal communication).
Current in dB re 1 mApeak was calculated using the formula:

Psychophysical procedures
Two psychophysical procedures were used: a method of adjustment and a two-interval forced-choice (2IFC) procedure with flanking cues coupled with a 2-down-1-up adaptive-tracking procedure. The method of adjustment was used to measure T levels and C levels. The procedure for the method of adjustment was similar to that used in fitting implants clinically. In this procedure, the stimuli were presented repeatedly with a 500-ms on/off duty cycle. A control knob for adjusting the level of the current was utilized. The subject was instructed to adjust the level of the signal until it was “barely audible.” First, T levels were determined in sequence from the apical electrodes to the basal electrodes. Then C levels were determined in the same direction. In order to check for adaptation to the test procedures, the T and C levels for the apical four stimulation sites were retested. If variation by more than 5 CL units was seen on the retest, the measurements were repeated. After determining T and C levels at each stimulation site, apical to basal sweeps were made to determine if any of the sites differed in loudness from the others. For this procedure, sweeps were made across overlapping subsets of 4–5 sites. Stimuli were 500 ms in duration with 500 ms between stimuli. Levels were adjusted, if necessary, for loudness balance. Later, a sweep across all sites was made. The first sweeps were made at the previously determined T levels, and the subjects were asked to be sure that each presentation in the sweep was heard and that all presentations were of equal loudness. Adjustments to T levels were made if needed and then the sweeps were repeated. Then sweeps at the C levels were presented. The subject was asked to be sure that none of the presentations was uncomfortably loud and that all presentations were of equal loudness. Adjustments to C levels were made if needed and then the sweeps were repeated. This process continued until the subject reported that all presentations were equally loud.
A 2IFC procedure with flanking cues was used to measure the TFC levels. Custom software presented four buttons labeled 1, 2, 3, and 4 from left to right on the computer screen. The buttons were illuminated in sequence for 500 ms each with a 500 ms interval between each. These buttons indicated the timing of the 4 observation intervals. Presentation of the electrical stimulus occurred during either interval 2 or interval 3, assigned at random. The other intervals contained no electrical stimulus. The subjects were instructed to use a computer mouse to point and select the button corresponding to the interval (interval 2 or interval 3) during which they heard the stimulus. The subjects were instructed to guess when they were not sure which interval had contained the stimulus. An adaptive 2-down-1-up procedure was used in which the stimulus amplitude was decreased by one step after two consecutive correct responses and was increased by one step after each incorrect response. The step size was 1 CL unit. The tracking was started 6 CL units above the T levels that had been measured previously with the method of adjustment. The procedure was run until 14 reversals were achieved. To estimate the TFC levels, the levels at the final eight reversals were averaged. This estimate corresponded to 70.7% correct responses (Levitt 1971).
RESULTS
Overview
Examples from 8 of the subjects of the C, T, and TFC levels obtained at each of the tested stimulation sites are shown in Figure 1. The examples in Figure 1 illustrate several important features of the data that are described quantitatively for data from all of the subjects in the following sections.
Examples of C, T, and TFC levels across stimulation sites for 8 subjects. The subject number is indicated in the lower left corner of each panel. Stimulation sites (number of the active electrode) are given on the abscissa with 1 being at the basal end and 21 near the apical end of the implant. The top row of panels shows data for four subjects with the CI24M implant and the bottom row shows data for four subjects with the CI24R(CS) implant. Black symbols and lines are used for BP stimulation and gray symbols and lines are used for MP stimulation. Filled triangles are used for T levels, open triangles for TFC levels, and filled circles for C levels. Triangles pointing toward the left ordinate indicate means of levels across the whole array.
Across-site variation
C levels and T levels showed a large range across all subjects and all stimulation sites: 31 dB for C levels and 34 dB for T levels with BP stimulation and 14 dB for C levels and 19 dB for T levels with MP stimulation. The distributions of C levels and T levels for all subjects at all tested sites are shown in Figure 2. The large range of T levels and C levels reflects a combination of variation across stimulation sites within subjects and across-subject variation. In this study we focused on across-site variation within subjects.
Distribution of C levels and T levels for CI24M (open symbols) and CI24R(CS) (filled symbols) implants. Data plotted with circles are for all tested stimulation sites in all subjects. For the 13 CI24M implant, there were data from 269 and 278 sites for BP and MP configurations, respectively. For the nine CI24R(CS) implants, there were data from 187 BP and 196 MP sites. Means of the distributions are indicated by the inverted triangles on the abscissa of each panel.
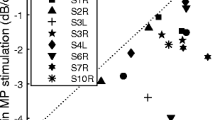
Site-to-site variation within subjects (mean of the absolute values of the differences for each electrode from its neighbor) is shown in Figure 3 where we compare site-to-site variation for MP and BP stimulation for each subject as discussed below.
Comparison of site-to-site variation in C, T, and TFC levels for BP stimulation with that for MP stimulation. Site-to-site variation was defined as the average absolute difference between each adjacent stimulation site in C, T, or TFC level (see Methods). Each circle represents an individual subject. Means of the distributions are indicated by the triangles on the axes. Open symbols are for CI24M implant and filled symbols are for the CI24R(CS) implant.
Effects of electrode configuration
For BP stimulation, there was a considerable range across subjects in the amount of site-to-site variation in T, TFC, and C levels. However, in almost all cases, site-to-site variation within subjects was larger for BP stimulation than for MP stimulation (Fig. 3, points above the diagonal). The only exception in this data set was one subject for which T levels for MP stimulation showed a slightly higher site-to-site variation than those for BP stimulation. Mean site-to-site variation values across subjects (triangles at the axes in Fig. 3) were 2.1–3.3 times larger for BP stimulation than for MP stimulation. These differences were statistically significant (paired t-tests, p < 0.001).
Lower site-to-site variation for MP stimulation was not due to the fact that the current levels for MP stimulation were lower than those for BP stimulation (i.e., it was not due to the scale used for T and C levels) because even when the levels for MP and BP stimulation were similar, the difference in across-site variation for BP versus MP could be large (e.g., for S12 in Fig. 1, compare C levels for MP stimulation with T levels for BP stimulation).
Across-site variation was also evaluated using two other within-subject metrics: range of T, TFC, and C levels across all stimulated sites and standard deviation of these levels across all stimulated sites. These metrics also showed significantly larger across-site variation for BP stimulation than for MP stimulation (paired t-tests, p < 0.001).
Effects of level
The C and T levels measured along the electrode array showed a similar pattern in most individual subjects (Fig. 1). To quantify the degree of similarity between the patterns of T and C level variations across the electrode arrays, we computed the standard deviations of the C and T level differences for each subject. For reference, consider the C and T patterns in the MP configuration, which were very similar to each other since both T and C levels showed little variation across the electrode array. The median of the standard deviations of the C and T differences for MP configuration for the 22 subjects was 1.87 dB. The C and T patterns in the BP configuration were also similar to each other. Although the site-to-site variation of the C and T levels for BP stimulation were high, the median of the standard deviations of the C and T differences for the BP configuration in the 22 subjects was only 2.66 dB. In two exceptional cases (S2 and S19), the across-site patterns of C levels in the BP configuration differed dramatically from those of the T levels. In these cases, the standard deviations of the C and T differences were 7.98 dB for S2 and 6.62 dB for S19 (data for S19 are shown in Fig. 1).
C levels and T levels for BP stimulation showed not only similar patterns of variation across stimulation sites, they also showed similar magnitudes of variation. This is shown in Figure 4, which compares site-to-site variation of C levels with that of T levels. For BP stimulation, statistical analysis revealed no significant differences in the ranges or site-to-site variation between C and T levels (paired t-tests, p > 0.0.5). For MP stimulation, however, site-to-site variation of T levels was higher than that for C levels in the majority of cases (Fig. 4, right panel) and the mean site-to-site variation for T levels was significantly higher than that for C levels (paired t-test, p < 0.01).
Comparison of site-to-site variation (as defined in Fig. 3) for C levels with that for T levels for BP and MP stimulation. Each circle represents an individual subject. Means of the distributions are indicated by the triangles on the axes. Open symbols are for the CI24M implant and filled symbols are for the CI24R(CS) implant.
Effects of level on across-site variation were also evaluated using the range of levels across all stimulated sites and standard deviation of levels across all stimulated sites. These metrics showed the same trends as the site-to-site variation metrics. However, neither measure showed a significant difference in across-site variation between C levels or T levels for BP or MP stimulation (paired t-tests, p > 0.05).
Effects of implant design
T levels and C levels for CI24M and CI24R(CS) implants showed considerable overlap (Fig. 2). In addition, it can be seen in Figure 3 that the range of site-to-site variation within subjects with the CI24M implant (open circles) overlapped that of the CI24R(CS) implant (filled circles). However, the distribution of variation data for the CI24M implant was skewed toward lower values whereas that for the CI24R(CS) implant was not. The means of site-to-site variation in C levels for the CI24M implant were significantly lower statistically than those for CI24R(CS) implant (paired t-tests, p < 0.05). For T levels, the means of site-to-site variation were also lower for the CI24M implant, but the differences from those for the CI24R(CS) implant were not statistically significant (paired t-test, p > 0.05).
Comparison of test–retest and across-site variation
In Session 2, effects of electrode configuration and stimulus level on variability of repeated measurements (test–retest variability) were compared with the effects of these variables on across-site variability. In general, we found that electrode configuration had a smaller and less consistent effect on test–retest variability than it did on across-site variability measured in the same session (Fig. 5A, B). For C levels, the ratio of standard deviations for data collected across stimulation sites using BP stimulation to that for MP stimulation was always greater than 1, ranging from 1.7 to 5.0, across subjects (Fig. 5A, abscissa). They indicate that across-site variation in C levels was always greater for BP stimulation than for MP stimulation in these subjects. In these same subjects, the ratio of standard deviations for repeated tests with BP stimulation to that for MP stimulation was also greater than 1 in 10 of the 13 cases, indicating that electrode configuration also had an effect on test–retest variation. However, when the BP/MP ratios of standard deviations for repeated measures were compared with these ratios for standard deviations across stimulation sites in the same subjects (Fig. 5A), we found that the ratios for across-site variation were larger in all cases. The mean BP/MP ratio for across-site standard deviations was significantly larger than that for test–retest standard deviations (paired t-test, p < 0.01), indicating that the effect of electrode configuration on across-site variation was greater than the effect of this variable on test–retest variation.
Comparison of effects of electrode configuration (top row) and stimulus level (bottom row) on test–retest standard deviations (ordinates) with the effects of these variables on across-site standard deviations (abscissas). Effects of electrode configuration are represented by the ratios of standard deviations for BP stimulation to those for MP stimulation. Effects of level are represented by the ratios of standard deviations for T levels to those for C levels. Medians of the distributions are indicated by the triangles on the abscissas and ordinates. The three outliers represent cases with very small standard deviations in the denominators.
A similar pattern was seen in the data from Session 2 for T levels (Fig. 5B). For these data, there were three cases where the BP/MP ratios for test–retest variation were larger than those for across-site variation (points above the diagonal), but for the most part the ratios for across-site variation were larger (points below the diagonal). For test–retest variation, the ratios ranged from 0.8 to 2.1 with a median of 1.4, while for across-site variation they ranged from 1.0 to 4.8 with a median of 2.6. The ratios for across-site variation were significantly larger than those for test–retest variation (paired t-test, p < 0.01).
For BP stimulation (Fig. 5C), the T/C ratios were close to 1 for both test-retest and across-site variation indicating that, on average, variation for T levels was similar to that for C levels. The effects of level on test–retest variation were not distinguishable from those for across-site variation (paired t-test, p > 0.05). Stimulus level had a small effect on both across-site and test–retest variation for MP stimulation (Fig. 5D). In both cases the median T/C ratio was greater than 1, indicating that across-site and test–retest variations were slightly lower on average at C levels compared with those at T levels. However, effects of level on test–retest variation were not distinguishable from those on across-site variation (paired t-test, p > 0.05).
Effects of psychophysical procedure
T levels were higher than TFC levels in 84.2% of cases with BP stimulation and 80.3% of cases with MP stimulation. On average, the 2IFC method yielded thresholds that were 1.9 dB lower than T levels for BP stimulation and 1.5 dB lower for MP stimulation. These differences corresponded to about 11 and 8 CL units, respectively. The differences between T and TFC levels were statistically significant (paired t-test, p < 0.01). Despite such differences, the pattern of site-to-site variation in TFC levels was similar to that for T levels (Fig. 1).
The site-to-site variation for the two psychophysical procedures did not differ significantly with either BP or MP configurations (paired t-tests, p > 0.05). As noted above, the site-to-site variations of TFC levels for the BP configuration were larger in all cases than those for the MP configuration (Fig. 3).
DISCUSSION
Mechanisms underlying across-site variation
The current study assessed across-site variation in T and C levels as a potential metric related to variation in the distance between the electrodes and the excitable neural elements and/or the spatial extent of neural activation. As noted in the Introduction, two models of the mechanisms underlying across-site variation and effects of electrode configuration were considered.
According to one model, which we will call the electrode–neuron-distance model, threshold for a given stimulation site depends on the length and condition of the pathway between the stimulation site (electrodes) and the excitable neural elements. The length of this pathway can be affected by the location of the electrodes within the scala tympani and by the condition of the neural population. For a narrow-bipolar electrode configuration, if the path from the stimulation sites to the most sensitive neurons is short, relatively little current will be required to excite the most sensitive neurons. If the most sensitive neurons are remote, more current will be required. Hence, across-site variation in T levels for narrow-bipolar stimulation will be large in cochleae where the relationship between the stimulation sites and the responsive neurons is not uniform across the electrode array. For a monopolar electrode configuration, the voltage gradient is shallow so that the current level will be similar at nearby and more remote sites of excitation. Hence, across-site variation in T levels for monopolar stimulation will be small. Electrode–neuron-distance models include one in which excitation of only one neuron is required to achieve stimulus detection, although these models are not limited to that condition.
An alternative model requires neural integration of activity across multiple activated neurons in order to achieve stimulus detection. We will call this the neural-integration model. Similar to the electrode–neuron-distance model, the neural-integration model assumes that variation in detection thresholds is a result of variation in the sensitivity of the neurons along the length of the scala tympani and/or variation in the current paths from the stimulation sites to the neurons. Conditions that produce large spatial extents of neural activation can achieve psychophysical detection thresholds at low current levels by integrating across a large number of neurons that are activated at low discharge rates (Bruce et al. 1999). Because the number of neurons activated at the detection threshold is large, the detection threshold will be based on the average of high-threshold and low-threshold neurons and will be similar to the average threshold of neurons activated by a nearby site, particularly if there is a large overlap in the activated neural populations. In contrast, conditions that produce spatially restricted activating-current fields will achieve detection threshold by activating only a restricted population of neurons, but it might require higher discharge rates in each of the activated neurons in order to produce the number of neuronal discharges required to achieve stimulus detection. Because these configurations achieve threshold by stimulating a more spatially restricted population of neurons, they will be more sensitive to local variations in the sensitivity of the neurons to electrical stimulation. If nearby subpopulations of neurons that are activated by different stimulation sites differ in sensitivity, then across-site variability will be high.
Both electrode configuration and stimulus level affect sizes of activating-current fields and the sizes of the activated neural populations. However, the predictions regarding effects of these independent variables on across-site variation depend on the model employed.
Now, consider the effects of current level under the electrode–neuron-distance model. When the current level is increased from the detection threshold level (T level) to the maximum comfortable loudness level (C level), there is certain to be an increase in the size of the activated neural population (Snyder et al. 1990; Bierer and Middlebrooks 2002). At the same time, the discharge rates of the most sensitive neurons, which were activated at threshold, will increase (Hartmann and Klinke 1990). Both the increase in number of neurons recruited and the increase in discharge rate of previously recruited neurons might lead to increases in loudness. However, the effect of the increase in spread of activation on across-site variation in C levels might be much different than the effect observed at the T levels for the following reason. Once the most sensitive neurons have been activated at the psychophysical detection threshold level, increases in stimulus level will have similar effects both for neurons that are close to the stimulation sites and/or most sensitive and for those that are more remote and/or less sensitive. Thus the across-site variations seen at the threshold level will be maintained at suprathreshold equal-loudness levels. Our data support this model.
In contrast to the electrode–neuron-distance model, the neural-integration model accounts for across-site variation in terms of the size of the activated neural population. If the across-site variation in the current required to reach just-detectable loudness (T levels) can be reduced by increasing the size of the neural population contributing to the threshold percept, then it follows that increasing the size of the neural population by increasing current level (and loudness) should also reduce across-site variation. Thus, variation at maximum-comfortable loudness level (C level) should be smaller than that at the threshold-loudness level (T level) for the same electrode array, provided that the measure of C levels is not inherently more variable than the measure of T levels. Our data from Session 2 showed that C levels were not more variable across repeated tests under the same conditions than were T levels (T/C ratios were usually slightly greater than 1). In Session 2 as well as in Session 1, for BP stimulation we found no consistent evidence of reduced across-site variation in C levels relative to that for T levels. Thus, for BP stimulation, the prediction of the neural-integration model was not supported by our data.
This is not to say that loudness per se does not depend on integration of activity across multiple neurons. Rather, the data simply indicate that across-site variation in the amount of current required to achieve a given loudness level (T or C) is not altered by increasing the number of neurons across which the activity is integrated. This in turn suggests that the mechanism by which electrode configuration affects across-site variation in C levels and T levels is not based on across-neuron integration. Thus, our data favor the electrode–neuron-distance model to account for across-site variation and to account for the effects of electrode configuration and level.
For MP stimulation, there was evidence for smaller across-site variation in C levels relative to T levels. Clearly, the effect of level on across-site variation for MP stimulation is not as large or as consistent as the effect of electrode configuration (compare Fig. 4 with Fig. 3). It is known that spread of neural excitation increases markedly with increases in stimulus level (Hartmann and Klinke 1990; Snyder et al. 1990; Bierer and Middlebrooks 2002). Given this and given the weak effect of level on across-site variation for MP stimulation and the lack of a consistent effect for BP stimulation, we conclude that spread of neural excitation at levels above the stimulus detection threshold has little effect on across-site variation.
We found a great deal of overlap in the distribution of BP and MP thresholds for the CI24M and the CI24R(CS) implants. The average values for these measures were in the expected direction, i.e., lower thresholds for the CI24R(CS) implant relative to the CI24M implant. However, these differences were relatively small. Other studies have also found small effects of implant type. The difference in mean MP thresholds for CI24M and CI24R(CS) implant groups found by Saunders et al. (2002) was 13.8 Cochlear Level units, equivalent to about 2.4 dB. In our study, this difference was 0.8 dB. The wide distribution of these values and the considerable overlap in the distributions for the two types of implants suggest that there is a great deal of variability from site to site in the distances between the electrodes and the neurons in both types of implants. For the CI24R(CS) implant, this variability is also evident in the distance between the electrodes and the modiolar core as assessed by analysis of radiographic images (Saunders et al. 2002). Another factor that would affect the variability in distance between the stimulated electrodes and the responsive neurons is the large variability in nerve survival patterns that is typically found in deaf ears (Hinojosa and Marion 1983; Nadol 1997). Even if electrodes are consistently placed near the medial wall of the scala tympani, there is no guarantee that there will be responsive neurons near all of the stimulation sites.
There was also a great deal of overlap in the distributions of site-to-site variation for the CI24M and CI24R(CS) implants. However, the site-to-site variation for the CI24M implant was lower on average (Fig. 3). These data are consistent with a more uniform positioning of the CI24M implant against the lateral wall of the scala tympani compared with the CI24R(CS) implant, which may vary in its medial–lateral position as suggested by the radiographic data of Saunders et al. (2002).
Control experiments
In order to interpret the effects of electrode configuration and stimulus level on across-site variation of thresholds and maximum comfortable loudness levels, it is necessary to know how these two independent variables affect threshold and maximum comfortable loudness measures in general. To assess this, in Session 2 we compared the effects of electrode configuration and stimulus level on across-site variation with their effects on variation over repeated measures (test–retest variation) for the same subjects under the same conditions. Although we did find small effects of electrode configuration on test–retest standard deviations, the effects on across-site standard deviations were more pronounced suggesting that there was a specific effect of electrode configuration on across-site variation.
Surprisingly, test–retest standard deviation was very similar for T levels and C levels indicating that subjects were equally reliable in their estimation of threshold and maximum comfortable loudness levels when they were calculated in decibels.
We found that TFC levels were slightly lower than T levels in the majority of cases, suggesting that, in general, subjects are a little conservative when estimating minimum detectable levels. However, importantly, we did not find a difference in across-site variation assessed by these two threshold measurement procedures. Thus it seems that the commonly used clinical procedure is adequate for assessing across-site variation.
Applications
Data from this study suggest that detection thresholds for BP stimulation might serve as a metric for determining the functional distance between the electrodes and the sites of action-potential initiation on a site-by-site basis. An advantage of a psychophysical measure over imaging measures of electrode-to-neuron distances is that it reflects the functional distances to the sites of neural activation, which cannot be assessed noninvasively with current imaging techniques. This measure might be useful for assessing the functional effects of techniques that are intended to decrease the functional distance between electrodes and neurons, including implant designs (Shepherd et al. 1993) and tissue engineering procedures intended to increase nerve survival and/or promote growth of neurons toward the electrodes (Miller et al. 2002). In addition, the measure might be useful for selecting the most effective sites for stimulation from a high-density electrode array.
The advantages or disadvantages of reducing electrode-to-neuron distance are poorly understood at the present time. Use of the across-site variation metrics in conjunction with other functional measures should help to clarify the relationship between implant and tissue engineering procedures and information transmission in cochlear prostheses. Understanding these relationships should provide useful guidance in determining optimal treatments and stimulation strategies for individual patients.
References
JA Bierer JC Middlebrooks (2002) ArticleTitleAuditory cortical images of cochlear-implant stimuli: Dependence on electrode configuration. J. Neurophysiol. 87 478–492 Occurrence Handle11784764
IC Bruce MW White LS Irlicht SJ O’Leary GM Clark (1999) ArticleTitleThe effects of stochastic neural activity in a model predicting intensity perception with cochlear implants: Low-rate stimulation. IEEE Trans. Biomed. Eng. 46 1393–1404 Occurrence Handle10.1109/10.804567 Occurrence Handle1:STN:280:DC%2BD3c%2FnvFOhsw%3D%3D Occurrence Handle10612897
PA Busby LA Whitford PJ Blamey LM Richardson GM Clark (1994) ArticleTitlePitch perception for different modes of stimulation using the cochlear multiple-electrode prosthesis. J. Acoust. Soc. Am. 95 2658–2669 Occurrence Handle1:STN:280:ByuB28jgvF0%3D Occurrence Handle8207139
NL Cohen JT Roland Jr A Fishman (2002) ArticleTitleSurgical technique for the Nucleus contour cochlear implant. Ear Hear. 23 59S–66S Occurrence Handle10.1053/euhj.2001.2690 Occurrence Handle11883768
MF Dorman PC Loizou J Fitzke Z Tu (1998) ArticleTitleThe recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6-20 channels. J. Acoust. Soc. Am. 104 3583–3585 Occurrence Handle10.1121/1.423940 Occurrence Handle1:STN:280:DyaK1M%2FnsVynsQ%3D%3D Occurrence Handle9857516
KE Fishman RV Shannon WH Slattery (1997) ArticleTitleSpeech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J. Speech Lang. Hear. Res. 40 1201–1215 Occurrence Handle1:STN:280:ByiH28bkt1w%3D Occurrence Handle9328890
LM Friesen RV Shannon D Baskent X Wang (2001) ArticleTitleSpeech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. J. Acoust. Soc. Am. 110 1150–1163 Occurrence Handle10.1121/1.1381538 Occurrence Handle1:STN:280:DC%2BD3Mvns1artw%3D%3D Occurrence Handle11519582
QJ Fu RV Shannon X Wang (1998) ArticleTitleEffects of noise and spectral resolution on vowel and consonant recognition: Acoustic and electric hearing. J. Acoust. Soc. Am. 104 3586–3596 Occurrence Handle10.1121/1.423941 Occurrence Handle1:STN:280:DyaK1M%2FnsVyntg%3D%3D Occurrence Handle9857517
R Hartmann R Klinke (1990) ArticleTitleImpulse patterns of auditory nerve fibers to extra- and intracochlear electrical stimulation. Acta Otolaryngol. Suppl. (Stockh.) 469 128–134
R Hinojosa M Marion (1983) ArticleTitleHistopathology of profound sensorineural deafness. Ann. NY Acad. Sci. 405 459–484 Occurrence Handle1:STN:280:BiyB2MvjsVE%3D Occurrence Handle6575668
A Kawano HL Seldon GM Clark RT Ramsden CH Raine (1998) ArticleTitleIntracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Otolaryngol. (Stockh.) 118 313–326
DW Kennedy (1987) ArticleTitleMultichannel intracochlear electrodes: Mechanism of insertion trauma. Laryngoscope 97 42–49
A Kral R Hartmann D Mortazavi R Klinke (1998) ArticleTitleSpatial resolution of cochlear implants: The electrical field and excitation of auditory afferents. Hear. Res. 121 11–28 Occurrence Handle10.1016/S0378-5955(98)00061-6 Occurrence Handle1:STN:280:DyaK1czks1egtg%3D%3D Occurrence Handle9682804
H Levitt (1971) ArticleTitleTransformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 49 467–477 Occurrence Handle5541744
JM Miller AL Miller T Yamagata G Bredberg RA Altschuler (2002) ArticleTitleProtection and regrowth of the auditory nerve after deafness: neurotrophins, antioxidants and depolarization are effective in vivo. Audiol. Neurootol. 7 175–179 Occurrence Handle10.1159/000058306 Occurrence Handle1:CAS:528:DC%2BD38XksVKkt7Y%3D Occurrence Handle12053141
J Nadol (1997) ArticleTitlePatterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol. Head Neck Surg. 117 220–228 Occurrence Handle9334769
AJ Parkinson J Arcaroli SJ Staller PL Arndt A Cosgriff K Ebinger (2002) ArticleTitleThe Nucleus 24 Contour Cochlear Implant System: Adult clinical trial results. Ear Hear. 23 41S–48S Occurrence Handle10.1053/euhj.2001.2694 Occurrence Handle11883766
BE Pfingst I Glass FA Spelman D Sutton (1985) Psychophysical studies of cochlear implants in monkeys: Clinical implications. RA Schindler MM Merzenich (Eds) Cochlear Implants. Raven Press New York 305–321
BE Pfingst TA Zwolan LA Holloway (1997) ArticleTitleEffects of stimulus configuration on psychophysical operating levels and on speech recognition with cochlear implants. Hear. Res. 112 247–260 Occurrence Handle10.1016/S0378-5955(97)00122-6 Occurrence Handle1:STN:280:DyaK1c%2FjtlSlug%3D%3D Occurrence Handle9367245
E Saunders L Cohen A Aschendorff W Shapiro M Knight M Stecker B Richter S Waltzman M Tykocinski T Roland R Laszig R Cowan (2002) ArticleTitleThreshold, comfortable level and impedance changes as a function of electrode modiolar distance. Ear Hear. 23 28S–40S Occurrence Handle11883764
RV Shannon F-G Zeng V Kamath J Wygonski M Ekelid (1995) ArticleTitleSpeech recognition with primarily temporal cues. Science 270 303–304 Occurrence Handle1:CAS:528:DyaK2MXoslehsL8%3D Occurrence Handle7569981
RK Shepherd GM Clark BC Pyman RL Webb (1985) ArticleTitleBanded intracochlear electrode array: Evaluation of insertion trauma in human temporal bones. Ann. Otol. Rhinol. Laryngol. 94 55–59 Occurrence Handle1:STN:280:BiqC38%2FltFU%3D Occurrence Handle3838226
RK Shepherd S Hatsushika GM Clark (1993) ArticleTitleElectrical stimulation of the auditory nerve: The effect of electrode position on neural excitation. Hear. Res. 66 108–120 Occurrence Handle10.1016/0378-5955(93)90265-3 Occurrence Handle1:STN:280:ByyB3s7ptFc%3D Occurrence Handle8473242
RL Snyder SJ Rebscher K Cao PA Leake K Kelly (1990) ArticleTitleChronic intracochlear electrical stimulation in the neonatally deafened cat. I. Expansion of central representation. Hear. Res. 50 7–33 Occurrence Handle10.1016/0378-5955(90)90030-S Occurrence Handle1:STN:280:By6C28bpslA%3D Occurrence Handle2076984
EL von Wallenberg R Laszig D Gnadeberg R Battmer C Desloovere J Kiefer E Lehnhardt C von Ilberg (1994) Initial findings with a modified Nucleus implant comprised of 20 active intracochlear and 2 extracochlear reference electrodes. IJ Hochmair–Desoyer ES Hochmair (Eds) Advances in Cochlear Implants. Manz Vienna 186–192
L Xu Y Tsai BE Pfingst (2002) ArticleTitleFeatures of stimulation affecting tonal-speech perception: Implications for cochlear prostheses. J. Acoust. Soc. Am. 112 247–258 Occurrence Handle10.1121/1.1487843 Occurrence Handle12141350
Acknowledgements
This work was supported by NIH/NIDCD Grants R01 DC 03808 and T32 DC00011. Our thanks to the human subjects who participated in this research, to Julie Oldfield and Cathy Thompson for assistance with data collection and analysis, and to John Middlebrooks for helpful discussions regarding these data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfingst, B.E., Xu, L. Across-Site Variation in Detection Thresholds and Maximum Comfortable Loudness Levels for Cochlear Implants . JARO 5, 11–24 (2004). https://doi.org/10.1007/s10162-003-3051-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-003-3051-0