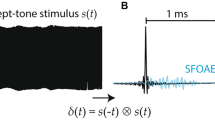
Abstract
According to coherent reflection theory (CRT), stimulus frequency otoacoustic emissions (SFOAEs) arise from cochlear irregularities coherently reflecting energy from basilar membrane motion within the traveling-wave peak. This reflected energy arrives in the ear canal predominantly with a single delay at each frequency. However, data from humans and animals indicate that (1) SFOAEs can have multiple delay components, (2) low-frequency SFOAE delays are too short to be accounted for by CRT, and (3) “SFOAEs” obtained with a 2nd (“suppressor”) tone ≥2 octaves above the probe tone have been interpreted as arising from the area basal to the region of cochlear amplification. To explore these issues, we collected SFOAEs by the suppression method in guinea pigs and time-frequency analyzed these data, simulated SFOAEs, and published chinchilla SFOAEs. Time-frequency analysis revealed that most frequencies showed only one SFOAE delay component while other frequencies had multiple components including some with short delays. We found no systematic patterns in the occurrence of multiple delay components. Using a cochlear model that had significant basilar membrane motion only in the peak region of the traveling wave, simulated SFOAEs had single and multiple delay components similar to the animal SFOAEs. This result indicates that multiple components (including ones with short delays) can originate from cochlear mechanical irregularities in the SFOAE peak region and are not necessarily indicative of SFOAE sources in regions ≥2 octaves basal of the SFOAE peak region. We conclude that SFOAEs obtained with suppressors close to the probe frequency provide information primarily about the mechanical response in the region that receives amplification, and we attribute the too-short SFOAE delays at low frequencies to distortion-source SFOAEs and coherent reflection from multiple cochlear motions. Our findings suggest that CRT needs revision to include reflections from multiple motions in the cochlear apex.








Similar content being viewed by others
References
Backus BC, Guinan JJ Jr (2006) Time course of the human medial olivocochlear reflex. J Acoust Soc Am 119:2889–2904
Boothalingam S, Lineton B (2012) Effect of contralateral acoustic stimulation on cochlear tuning measured using stimulus frequency and distortion product OAEs. Int J Audiol 51:892–9
Charaziak KK, Siegel JH (2014) Estimating cochlear frequency selectivity with stimulus-frequency otoacoustic emissions in chinchillas. J Assoc Res Otolaryngol 15:883–896
Charaziak KC, Siegel JH (2015a) Short-latency SFOAE components extracted with different methods. J Assoc Res Otolaryngol 38:169, #282
Charaziak KK, Siegel JH (2015b) Tuning of SFOAEs evoked by low-frequency tones is not compatible with localized emission generation. J Assoc Res Otolaryngol 16:317–29
Charaziak KK, Souza P, Siegel JH (2013) Stimulus-frequency otoacoustic emission suppression tuning in humans: comparison to behavioral tuning. J Assoc Res Otolaryngol 14:843–862
Chen F, Zha D, Fridberger A, Zheng J, Choudhury N, Jacques SL, Wang RK, Shi X, Nuttall AL (2011) A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci 14:770–774
Choi YS, Lee SY, Parham K, Neely ST, Kim DO (2008) Stimulus-frequency otoacoustic emission: measurements in humans and simulations with an active cochlear model. J Acoust Soc Am 123:2651–2669
Geisler CD, Yates GK, Patuzzi RB, Johnston BM (1990) Saturation of outer hair cell receptor currents causes two-tone suppression. Hear Res 44:241–256
Goodman SS, Withnell RH, Shera CA (2003) The origin of SFOAE microstructure in the guinea pig. Hear Res 183:7–17
Guinan JJ Jr (1990) “Changes in stimulus frequency otoacoustic emissions produced by two-tone suppression and efferent stimulation in cats”. In: Dallos P, Geisler CD, Matthews JW, Steele CR (eds) Mechanics and biophysics of hearing. Springer, Madison, WI, pp 170–177
Guinan JJ Jr (2012) How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res 292:35–50
Guinan JJ Jr, Cooper NP (2008) Medial olivocochlear efferent inhibition of basilar-membrane responses to clicks: evidence for two modes of cochlear mechanical excitation. J Acoust Soc Am 124:1080–1092
Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V (2003) Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol 4:521–540
Guinan JJ Jr, Lin T, Cheng H (2005) Medial-olivocochlear-efferent inhibition of the first peak of auditory-nerve responses: evidence for a new motion within the cochlea. J Acoust Soc Am 118:2421–2433
Joris PX, Bergevin C, Kalluri R, Mc Laughlin M, Michelet P, van der Heijden M, Shera CA (2011) Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in humans. Proc Natl Acad Sci U S A 108:17516–17520
Kalluri R, Shera CA (2001) Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am 109:622–637
Kalluri R, Shera CA (2007) Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 121:2097–2110
Kalluri R, Shera CA (2013) Measuring stimulus-frequency otoacoustic emissions using swept tones. J Acoust Soc Am 134:356–368
Keefe DH, Ellison JC, Fitzpatrick DF, Gorga MP (2008) Two-tone suppression of stimulus frequency otoacoustic emissions. J Acoust Soc Am 123:1479–94
Kiang, NYS (1984) Peripheral neural processing of auditory information. In: Handbook of physiology, section 1: the nervous system, Vol. 3 (Sensory processes). American Physiological Society, Bethesda, MD, pp. 639–674
Lewis, J D, Goodman, S S (2014a). Basal contributions to short-latency transient-evoked otoacoustic emission components. J Assoc Res Otolaryngol. 1-17
Lewis JD, Goodman SS (2014b) The effect of stimulus bandwidth on the nonlinear-derived tone-burst-evoked otoacoustic emission. J Assoc Res Otolaryngol 15:915–931
Lichtenhan JT, Cooper NP, Guinan JJ Jr (2013) A new auditory threshold estimation technique for low frequencies: proof of concept. Ear Hear 34:42–51
Moleti A, Longo F, Sisto R (2012) Time-frequency domain filtering of evoked otoacoustic emissions. J Acoust Soc Am 132:2455–2467
Moleti A, Mohsin Al-Maamury A, Bertaccini D, Botti T, Sisto R (2013) Generation place of the long- and short-latency components of transient-evoked otoacoustic emissions in a nonlinear cochlear model. J Acoust Soc Am 133:4098–4108
Nowotny M, Gummer AW (2006) Nanomechanics of the subtectorial space caused by electromechanics of cochlear outer hair cells. Proc Natl Acad Sci U S A 103:2120–2125
Palmer AR, Shackleton TM (2009) Variation in the phase of response to low-frequency pure tones in the guinea pig auditory nerve as functions of stimulus level and frequency. J Assoc Res Otolaryngol 10:233–250
Pfeiffer RR, Molnar CE (1970) Cochlear nerve fiber discharge patterns: relationship to the cochlear microphonic. Science 167:1614–1616
Rhode WS (2007) Basilar membrane mechanics in the 6–9 kHz region of sensitive chinchilla cochleae. J Acoust Soc Am 121:2792–2804
Ronken DA (1986) Anomalous phase relations in threshold-level responses from gerbil auditory nerve fibers. J Acoust Soc Am 79:417–425
Ruggero MA, Rich NC, Recio A, Narayan SS, Robles L (1997) Basilar-membrane responses to tones at the base of the chinchilla cochlea. J Acoust Soc Am 101:2151–2163
Shera CA, Bergevin C (2012) Obtaining reliable phase-gradient delays from otoacoustic emission data. J Acoust Soc Am 132:927–943
Shera CA, Guinan JJ Jr (1999) Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am 105:782–798
Shera CA, Guinan JJ Jr (2003) Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am 113:2762–2772
Shera CA, Guinan JJ Jr, Oxenham AJ (2002) Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci U S A 99:3318–3323
Shera C A, Tubis A, Talmadge C L, Guinan J J, Jr. (2004). The dual effect of “suppressor” tones on stimulus-frequency otoacoustic emissions. Assoc Res Otolaryngol Abstr 27, Abs. 776
Shera CA, Tubis A, Talmadge CL (2008) Testing coherent reflection in chinchilla. J Acoust Soc Am 123:3851
Shera CA, Guinan JJ Jr, Oxenham AJ (2010) Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol 11:343–365
Siegel JH, Temchin AN, Ruggero M (2003) Empirical estimates of the spatial origin of stimulus-frequency otoacoustic emissions. Assoc Res Otolaryngol Abstr 26:172 (#679)
Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero M (2005) Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am 118:2434–2443
Sisto R, Sanjust F, Moleti A (2013) Input/output functions of different-latency components of transient-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am 133:2240–2253
Sisto R, Moleti A, Shera CA (2015) On the spatial distribution of the reflection sources of different latency components of otoacoustic emissions. J Acoust Soc Am 137:768
Talmadge CL, Tubis A, Long GR, Tong C (2000) Modeling the combined effects of basilar membrane nonlinearity and roughness on stimulus frequency otoacoustic emission fine structure. J Acoust Soc Am 108:2911–2932
Temchin AN, Ruggero MA (2010) Phase-locked responses to tones of chinchilla auditory nerve fibers: implications for apical cochlear mechanics. J Assoc Res Otolaryngol 11:297–318
Temchin AN, Recio-Spinoso A, van Dijk P, Ruggero MA (2005) Wiener kernels of chinchilla auditory-nerve fibers: verification using responses to tones, clicks and noise and comparison with basilar-membrane vibrations. J Neurophysiol 93:3635–48
Temchin AN, Rich NC, Ruggero MA (2008) Threshold tuning curves of chinchilla auditory-nerve fibers. I. Dependence on characteristic frequency and relation to the magnitudes of cochlear vibrations. J Neurophysiol 100:2889–98
Tsuji J, Liberman MC (1997) Intracellular labeling of auditory nerve fibers in guinea pig: central and peripheral projections. J Comp Neurol 381:188–202
Versnel H, Prijs VF, Schoonhoven R (1990) Single-fibre responses to clicks in relationship to the compound action potential in the guinea pig. Hear Res 46:147–160
Zha D, Chen F, Ramamoorthy S, Fridberger A, Choudhury N, Jacques SL, Wang RK, Nuttall AL (2012) In vivo outer hair cell length changes expose the active process in the cochlea. PLoS One 7, e32757
Zweig G, Shera CA (1995) The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am 98:2018–2047
Acknowledgments
We thank Drs. C.A. Shera, M.C. Brown, D.C. Mountain, and K. K. Charaziak for comments on earlier versions of the manuscript. This work was supported by RO1 DC000235, R01 DC003687, T32 DC00038, P30 DC005209, and a NSF GRFP, and was part M. B-G’s PhD thesis.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berezina-Greene, M.A., Guinan, J.J. Stimulus Frequency Otoacoustic Emission Delays and Generating Mechanisms in Guinea Pigs, Chinchillas, and Simulations. JARO 16, 679–694 (2015). https://doi.org/10.1007/s10162-015-0543-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-015-0543-7