Abstract
Cochlear implant listeners receive auditory stimulation through amplitude-modulated electric pulse trains. Auditory nerve studies in animals demonstrate qualitatively different patterns of firing elicited by low versus high pulse rates, suggesting that stimulus pulse rate might influence the transmission of temporal information through the auditory pathway. We tested in awake guinea pigs the temporal acuity of auditory cortical neurons for gaps in cochlear implant pulse trains. Consistent with results using anesthetized conditions, temporal acuity improved with increasing pulse rates. Unlike the anesthetized condition, however, cortical neurons responded in the awake state to multiple distinct features of the gap-containing pulse trains, with the dominant features varying with stimulus pulse rate. Responses to the onset of the trailing pulse train (Trail-ON) provided the most sensitive gap detection at 1,017 and 4,069 pulse-per-second (pps) rates, particularly for short (25 ms) leading pulse trains. In contrast, under conditions of 254 pps rate and long (200 ms) leading pulse trains, a sizeable fraction of units demonstrated greater temporal acuity in the form of robust responses to the offsets of the leading pulse train (Lead-OFF). Finally, TONIC responses exhibited decrements in firing rate during gaps, but were rarely the most sensitive feature. Unlike results from anesthetized conditions, temporal acuity of the most sensitive units was nearly as sharp for brief as for long leading bursts. The differences in stimulus coding across pulse rates likely originate from pulse rate-dependent variations in adaptation in the auditory nerve. Two marked differences from responses to acoustic stimulation were: first, Trail-ON responses to 4,069 pps trains encoded substantially shorter gaps than have been observed with acoustic stimuli; and second, the Lead-OFF gap coding seen for <15 ms gaps in 254 pps stimuli is not seen in responses to sounds. The current results may help to explain why moderate pulse rates around 1,000 pps are favored by many cochlear implant listeners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cochlear implants restore hearing by stimulating the auditory nerve with amplitude-modulated electric pulse trains. In animal studies, pulse rates slower than ∼1,000 pulses per second (pps) elicit highly synchronous auditory nerve firing (e.g., Zhang et al. 2007). Much higher rates have been hypothesized to decrease neural synchrony, thereby better approximating the responses of the auditory nerve to sounds (Rubinstein et al. 1999). Some human listeners prefer high pulse rates (Battmer et al. 2010), but differences in speech recognition performance generally are small across pulse rates ranging from 400 to 4,800 pps (Loizou et al. 2000; Holden et al. 2002; Verschuur 2005; Plant et al. 2007; Shannon et al. 2011).
Gap detection is a measure of temporal acuity. Listeners attempt to detect a silent gap lying between leading and trailing stimuli that activate the same (within channel) or different (across channel) peripheral channels. Normal-hearing listeners can detect within-channel gaps as brief as 2–5 ms. Cochlear implant listeners can detect gaps of similar duration in electrical pulse trains (e.g., Shannon 1989). That gap thresholds are similar between normal-hearing listeners and cochlear implant users, in whom cochlear mechanotransduction is absent, suggests that gap detection thresholds are determined largely by retrocochlear (i.e., central to the cochlea) auditory mechanisms. Elevated gap detection thresholds are symptomatic of poor speech recognition in acoustic hearing, at least in cases of auditory neuropathy (Zeng et al. 2005), and there is some indication that in cochlear implant listeners impaired gap detection is associated with poor speech reception (Muchnik et al. 1994; Sagi et al. 2009).
Several studies have demonstrated parallels between perceptual gap detection thresholds and those measured in the cortex. In a study in humans, gap detection thresholds for leading markers of 5, 20, and 50 ms measured with magnetoencephalography were within 1 ms of those measured psychophysically (Rupp et al. 2004). In awake animals, 74% of cortical neurons in cat (Liu et al. 2010) and 25% in macaque (Recanzone et al. 2011) encoded gap duration with sufficient sensitivity to account for behavioral performance in those species.
We reported previously the responses of auditory cortex neurons in anesthetized guinea pigs to cochlear implant pulse trains (Kirby and Middlebrooks 2010). The onset of a leading pulse train consistently elicited a burst of spikes and, following a gap of sufficient duration, the trailing pulse train elicited a second burst of spikes. A period of suppression lasting ∼100 ms after the onset response blocked detection of gaps presented in that period. We suspected that the highly stereotyped patterns of responses in that study were due largely to the use of anesthesia.
The present study was conducted in awake, nonbehaving guinea pigs. We observed a variety of features of cortical responses that could signal the presence of a gap, and those responses varied with stimulus pulse rate, current level, and short versus long leading pulse-train duration. We confirmed our previous observation from anesthetized conditions that temporal acuity increased with increasing electrical pulse rate. We further tested the hypothesis that differences across pulse rate were attributable to auditory nerve adaptation by comparing gap detection thresholds for gaps lying early and late in the pulse-train stimulus. Finally, we tested the hypothesis that, as with human psychophysical measures, cortical gap detection thresholds decrease with increasing stimulus level.
This study provides a basis for understanding the ways in which pulse rate shapes the coding of cochlear implant stimulus envelopes. The effects of carrier pulse rate persist beyond the periphery as auditory nerve fiber responses are transformed by the central auditory system.
Methods
Overview
Extracellular spike activity was recorded from the primary auditory cortex (area A1) of awake, nonbehaving guinea pigs using chronic multi-electrode recording probes. Each animal underwent a surgical procedure to implant a recording probe in the right auditory cortex, with placement guided by cortical frequency tuning to acoustic tones. After suitable placement, the probe was fixed in place. The animal was then deafened bilaterally and a cochlear implant was inserted into the left cochlea (contralateral to the cortical recording sites). Recording sessions lasted 1–2 h and took place 3–8 days after the surgical procedure. All experiments were performed with the approval of the University of California at Irvine Institutional Animal Care and Use Committee.
Animal preparation
Recordings were made in the primary auditory cortex in the right hemispheres of six adult albino guinea pigs. Animals were of either sex and weighed 340–490 g at the time of surgery. The implantation procedure was performed under sterile conditions.
To eliminate the possibility of unintended acoustic stimulation, the right ear was deafened by infusion of 10% neomycin sulfate into the scala tympani (Middlebrooks 2004). The auditory cortex was then exposed on the right side. Primary auditory cortex was identified by its proximity to the pseudosylvian sulcus and by the characteristic rostrolateral to dorsomedial increase in characteristic frequencies, verified by recordings at three or more probe positions. When a cortical location within A1 with a characteristic frequency between 8 and 32 kHz had been identified, the recording probe was inserted perpendicular to the surface of the cortex. This frequency range corresponded approximately to the tonotopic location of the cochlear implant to be placed in the basal turn of the cochlea. The exposed dural surface and silicon shank of the recording probe were covered with a calcium alginate gel (Nunamaker et al. 2007). The flexible ribbon cable connecting the probe and connector was encased in silicone elastomer (World Precision Instruments, Sarasota, FL, USA). The connector was fixed to the skull with methacrylate cement. A screw was placed in the skull near the craniotomy to serve as a recording reference.
The left bulla was opened to expose the cochlea. A cochleostomy was drilled in the basal turn of the scala tympani and the ear was deafened with neomycin. A cochlear implant was inserted through the cochleostomy into the scala tympani. The cochlear implant was an eight-electrode banded array (Cochlear Corporation), identical in dimensions to the distal 8 electrodes of the clinical Nucleus 22. Only five bands could be placed within the scala tympani in our guinea pig preparation. The implant was sealed in place with biocompatible carboxylate cement (3 M ESPE, St. Paul, MN, USA). A platinum–iridium wire was placed into a neck muscle to serve as a monopolar return contact. All connectors were encased in methacrylate cement. The skin was closed around the two (stimulating and recording) percutaneous connectors. The animal was allowed to recover with supportive care.
Awake recording and data acquisition
Stimulus generation and data acquisition employed System III hardware from Tucker-Davis Technologies (TDT; Alachaua, FL, USA) coupled to a personal computer running MATLAB (The Mathworks, Natick, MA, USA) and using software developed in this laboratory. Pulsatile electric stimuli were generated by a custom eight-channel optically isolated current source controlled by the TDT hardware.
Recording sessions took place 3–8 days after surgery. Data presented from each animal were taken during one or two consecutive 1–2.5 h recording sessions, with 2.5–6 h elapsing between the first and last recordings. The order of stimulus presentation was different in each animal. Prior to recording, the recording electrodes were “rejuvenated” by passing 2.0 VDC through each electrode for 4 s to improve the signal-to-noise ratio (Johnson et al. 2004). Animals were lightly restrained with a Guinea Pig Snuggle (Lomir Biomedical, l’Ile Perrot, Quebec, Canada). Their heads were free, and they were monitored during the recording session to ensure that they did not sleep, struggle, or show signs of distress.
The cortical recording probe (NeuroNexus, Ann Arbor, MI, USA) was a single silicon substrate shank with 16 iridium-plated recording sites at 100-μm intervals. The probe was 15 μm thick and 100 μm wide, tapering to the tip; a monolithic silicon substrate ribbon cable carried the signals to the skull-mounted connector. Signals from all 16 recording sites were recorded simultaneously, digitized at 24.4 kHz, low-pass filtered, resampled at 12.2 kHz, and stored for offline analysis.
One of two artifact-rejection procedures was used to eliminate electrical artifact resulting from the electrical cochlear stimulus. For 254 pps stimuli, a sample-and-hold procedure was implemented such that the recorded neural signal was clamped at the onset of the electrical pulse, and recording resumed shortly after the pulse. In some cases in which voltage drift occurred between the onset and offset of the hold period, we interpolated the sampled voltage values between hold onset and onset. For 1,017 and 4,069 pps stimuli, artifact was removed with a comb filter tuned to the stimulus pulse rate. The comb filters eliminated artifact elicited by all but the first and last pulses of each stimulus, which were easily excluded from further analysis.
Online, a simple peak picker was used to monitor unit activity for experimental control. Offline, spikes were identified using custom software that classified waveforms on the basis of the time and amplitude differences between spike peaks and troughs. Only the offline-sorted spikes were used for quantitative analysis. Most channels provided unresolved spikes from two or more neurons. In this paper, a “unit” refers to the aggregate of spikes identified on a single recording channel.
Current source density analysis (Müller-Preuss and Mitzdorf 1984) was used to estimate the locations of recording sites relative to cortical layers. The most consistent feature was a short latency sink which we have found previously in histological reconstructions to correspond to the transition from layers III to IV (Middlebrooks 2008a). That landmark provided a depth reference that could be compared across animals.
Stimuli
The data required to compute an individual detection threshold or gap detection threshold were collected in a single block of trials, and several measurements were obtained from each block. For example, gap detection stimuli were varied over presentation level and gap duration to obtain gap detection thresholds for each of the presentation levels. Each configuration of stimulus parameters was repeated 20 (4 animals) or 40 (2 animals) times, and within each repetition the stimuli were interleaved randomly. Trials in which movement artifact was present in any recording trace, usually as a result of the animal chewing, were eliminated offline. At least 85% of trials were usable in 95% of conditions; the minimum was 13 trials in one condition. The order of testing of various parameters was varied among experiments.
Cochlear implant stimuli were presented in a monopolar electrode configuration in which a single intracochlear electrode was active and a wire in a neck muscle was the return. Thresholds for activation of the auditory cortex were estimated for each cochlear implant electrode and for each electrical pulse rate, and the electrode with the lowest threshold (always one of the two most apical electrodes) was selected for subsequent stimulation. Biphasic stimulus pulses were 41 μs per phase in duration with no interphase gap and with cathodic leading phase. The pulse rates tested were 254, 1,017, and 4,069 pps.
Electric gap detection stimuli consisted of pairs of electric pulse trains (“markers”) with identical pulse rate and current level separated by a pulse-free gap that varied in duration. All pulse-train durations and gaps were integer multiples of 0.246 ms, which is the period of the 4,069 pps. The duration of the leading marker was 23.6 or 196.6 ms. Gap duration was defined relative to the first missed pulse, such that that a gap of “0 ms” corresponded to an uninterrupted pulse train. Gaps that were shorter than the pulse period of the 254 or 1,017 pps pulse rates were achieved by delaying the entire trailing pulse train. Seven to 15 gap durations were presented in the ranges 0.492–256 ms for 254 pps and 0.246–256 ms for 1,017 and 4,069 pps pulse trains. Three sample stimuli are shown in Figure 1, at the bottom of each panel. Gap detection stimuli were presented at three current levels, most often 2, 4, and 6 dB above the most common threshold among the 16 recording sites. During blocks of gap detection trials, pulse rates and leading and trailing marker durations were fixed, but stimulus intensity and gap duration varied from trial to trial. Each block of trials yielded one gap detection threshold per stimulus level.
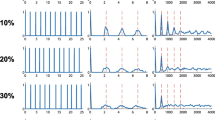
Gap detection stimuli, cortical responses, and analysis. At the bottom of each panel is a diagram of the 1,017 pps pulse train. Above is a raster plot in which each dot represents a spike and each row a stimulus presentation. The shaded areas represent analysis windows for the spike count ROC analysis. Gold shading TONIC window, blue shading short-latency ON window, green shading post-ON suppression window, purple shading long-latency ON window. At top, the summed PSTHs of these responses. A An early gap 132 ms in duration. B A 34-ms gap. C An uninterrupted pulse train, or 0 ms gap, shown with the analysis windows for the 132 ms gap.
Data analysis
Current-level detection thresholds based on neural spike counts were computed offline using signal detection procedures (Green and Swets 1966; Macmillan and Creelman 2005); the specific procedures are described by Middlebrooks and Snyder (2007). Briefly, receiver operating characteristic (ROC) curves were formed from the distribution of spike counts in trials that contained a stimulus and in trials that contained no stimulus. The area under the ROC curve gave the proportion of correct detections, which was expressed as a detection index (d′). Plots of d′ versus stimulus level were interpolated to find thresholds at d′ = 1. This procedure was followed for stimuli presented at each pulse rate. Detection thresholds were obtained for pulse trains 196.6 ms in duration, the same as the leading pulse train in the late-gap condition. The windows for spike rate analysis were selected to include the phasic ON response to the pulse train, as this response was elicited by the lowest current levels. Detection thresholds decreased systematically with increasing pulse rate. Across all units, median detection thresholds were −14.0, −14.4, and −16.9 dB re 1 mA for 254, 1,017, and 4,069 pps, respectively.
Three estimates of gap detection threshold were computed for each unit and each stimulus condition based on ROC analysis of spike count distributions within each of three time windows. Those windows corresponded to the onset of the trailing pulse train (the Trail-ON window), the offset of the leading pulse train (the Lead-OFF window), and a period of suppression of the tonic response between the two other windows (the TONIC window). In each case, trial-by-trial spike counts within a particular time window were compared between responses to stimuli that did or did not contain a gap—the time window was adjusted to accommodate each gap duration. For each gap duration, an ROC curve was formed from the distributions of spike counts in gap and no-gap conditions, and d′ was computed from the area under the ROC curve. An alternative to comparing absolute spike counts between gap and no-gap stimulus conditions would have been to compare spike rates across various portions of the response to a single stimulus. Spike rates varied throughout the duration of any stimulus, however, regardless of the presence or absence of a gap. For that reason, it would have been necessary to compute spike rate increments (or decrements) at particular post-stimulus onset times relative to some measure of baseline spike rate and, then, to compare the magnitudes of the increments or decrements between stimulus conditions. That procedure might have provided a form of normalization across varying overall spike rates. Nevertheless, in a trial-by-trial analysis of fairly sparse spike patterns, the need to estimate the baseline in addition to estimating the response to the gap in a particular time window would have introduced an additional source of trial-by-trial variance in the measure of response magnitude.
The d′ values obtained from comparison of spike counts in gap and no-gap conditions were linearly interpolated between tested gap durations. The shortest interpolated duration at which d′ = 1 (for onset and offset responses) or d′ = −1 (for post-onset suppression and tonic responses) was taken as the gap detection threshold. Responses visible in the aggregate data, such as the Trail-ON response to 0.5 ms gap in Figure 2C, sometimes occurred during individual trials of gap durations shorter than the calculated gap detection threshold. Those responses represent trial-by-trial variance that did not meet the d′ = 1 criterion in the ROC analysis of all the trials. Whereas all gaps presented were integer multiples of the 4,069 pps carrier pulse period, interpolated gap detection thresholds could fall anywhere between the minimum and maximum gaps tested.
Peri-stimulus time histogram of one unit to late-gap detection stimuli presented at 6 dB re stimulus threshold. Shaded areas represent the durations of leading and trailing markers. The height of each row corresponds to an instantaneous spike rate of 1,000 spikes/s. The vertical line corresponds to the latency of the peak of the OFF response. A 254-pps carrier rate with best gap detection threshold of 5.0 ms. The red outline demarcates the OFF response window. B 1,017-pps carrier rate with best gap detection threshold of 0.43 ms. C 4,069-pps carrier rate with best gap detection threshold of 0.6 ms.
Selection of time windows was crucial to the performance of the spike count measure because the spike patterns elicited by the gap stimuli varied from unit-to-unit as well as with stimulus level. Figure 1 illustrates the analysis windows chosen for one unit. Phasic ON responses were elicited by the onset of the leading marker and, in the presence of gaps of sufficient duration, about 5–30 ms after the onset of the trailing marker. For some units, such as the one in Figure 1, this short-latency ON response to the onset of the trailing marker was followed by a period of suppression, which was followed by a late-ON response. Thus, for some units, the spikes elicited by the onset of a trailing marker could be counted in up to three non-overlapping time windows. Figure 1 shows, for one unit, a short-latency ON window (5–16 ms, shaded in blue), a post-onset suppression window (16–30 ms, shaded in green), and a long-latency ON window (30–80 ms, shaded in purple), all measured relative to the onset of the trailing marker. For each unit, the gap detection threshold was computed for each of these windows, and the shortest of these gaps was chosen as the Trail-ON gap detection threshold. OFF responses, as seen in Figures 2 and 3, generally had a longer latency, with most OFF responses occurring 10–30 ms (outlined in red in Figure 2A) after the offset of the leading and/or trailing marker. Longer OFF-response latencies, 30–120 ms, were observed in 3% of units for stimuli at any pulse rate, and some OFF responses exhibited longer durations, with >100 ms of enhanced firing rate. The same OFF response window was used for all gap durations within a particular unit and stimulus level. The gap detection threshold computed for this window was the Lead-OFF gap detection threshold. TONIC responses, evident in Figure 1, were the third type of response characterized with this spike count measure. This analysis window (shaded in yellow) generally fell between the end of the offset window and the beginning of the short-latency onset window and measured the reduction in tonic firing that sometimes was observed during a gap in the stimulus. In the unit in Figure 1, the tonic firing is derived from the long-latency onset response to the leading pulse train. This analysis provided the TONIC gap detection threshold. These three measures (Trail-ON, Lead-OFF, and TONIC gap thresholds) were based on spike timing only and might have included spikes from multiple neurons.
Peri-stimulus time histogram of one unit’s responses to early-gap detection stimuli presented at 6 dB re stimulus threshold. Shaded areas represent the durations of leading and trailing pulse trains. The height of each row corresponds to an instantaneous spike rate of 1,000 spikes/s. A 254-pps carrier rate with best gap detection threshold of 6.6 ms B 1,017-pps carrier rate with best gap detection threshold of 4.0 ms C 4,069-pps carrier rate with best gap detection threshold of 3.4 ms.
The latency and duration of each time window for the measurement of Trail-ON and Lead-OFF responses were selected manually under visual guidance, but selection was informed by characteristics of responses throughout the entire duration of the stimuli to avoid overfitting. Windows were restricted to time ranges that were appropriate for responses elicited by all epochs of the stimulus, even those that were not informative about gaps. For instance, the onset of the leading marker evoked an ON response, which helped to verify the Trail-ON time-window selection. This decreased the likelihood of inadvertently selecting time windows to capture isolated fluctuations in firing rate. In some units, however, response timing varied with gap duration, such as the long-latency Trail-ON responses for 8–25 ms gaps in Figure 2B, and analysis windows were chosen to accommodate these responses. Each window was at least 10 ms in duration to avoid overfitting.
For sufficiently short gaps, Lead-OFF and Trail-ON response windows overlapped. In this case, spike latencies and rates were used to assign a particular response to the appropriate category. For instance, in Figure 2A, the response to the 8 ms gap was consistent either with a Lead-OFF or Trail-ON response. However, the unambiguous response to a 16-ms gap contained a Lead-OFF response and no Trail-ON response. Therefore, the significant response to the 8-ms gap was attributed to an OFF response and the ON-derived gap detection threshold was selected at the next upward d′ = 1 transition in the interpolated gap-d′ curve, 26 ms. In contrast, Figure 2B displays a response to gaps shorter than 2 ms that is most consistent with the spike count and latency of an ON response. Although the response to an 8-ms gap could once again be considered ambiguous, the response to a 12-ms gap clearly shows a superposition of Lead-OFF (shorter latency) and Trail-ON (longer latency) responses—in other words, more spikes were elicited for the longer duration gap. Therefore, the Lead-OFF gap detection threshold in such cases was derived by interpolating the shortest gap at which the ambiguous response differed in spike count and latency from the ON response. In this case, the gap detection threshold fell between 8 and 12 ms. Responses consistent with Lead-OFF spike rate and latency were never seen following a Trail-ON response, so this procedure generally resulted in a gap detection threshold determined by the difference between the latencies of Lead-OFF and Trail-ON responses. Finally, in Figure 2C there is no significant OFF response at the longest, unambiguous gap durations, so no OFF-derived gap detection threshold was obtained even though spikes fell within the offset analysis window. This procedure was followed for each unit in the sample.
Results
Responses to pulse trains containing gaps
Neurons in cortical area A1 responded to cochlear implant electrical pulse trains with phasic bursts of spikes associated with onsets and offsets of pulse trains and with sustained firing throughout the duration of the pulse train. The first example is shown in Figure 1, which shows the spikes elicited at one recording site for two early-gap stimuli and one control no-gap stimulus; the pulse rate was 1,017 pps. In response to a continuous pulse train (Figure 1C), this unit produced a burst of spikes 5–16 ms after the stimulus onset, followed by a 15-ms pause, followed by sustained firing that gradually decreased over the course of the stimulus. In Figure 1A and B, the ON response was observed after the onset of the leading pulse train and after the onset of pulse trains trailing the gaps. In this example, an ON response to the trailing stimulus was sufficient to reveal the presence of a gap.
Cortical response patterns differed according to the temporal position of the gap within the pulse train, the pulse rate, and the gap duration. Figure 2 represents the responses recorded on a single site for the late-gap condition and pulse rates of 254 pps (Figure 2A), 1,017 pps (Figure 2B), and 4,069 pps (Figure 2C). At the lowest pulse rate and longest gap duration (Figure 2A, 63-ms gap), both the leading and trailing pulse trains elicited an ON response with 5–20 ms latency, an OFF response with a 10–30 ms latency, and a weak TONIC response sustained for the duration of the pulse train. ON responses to the leading pulse train were present at all pulse rates and for all gap durations, whereas ON responses to the trailing pulse train were present only for gap durations of sufficient length. In contrast, OFF responses were evoked by the leading pulse train only for the lower pulse rates and the longest gap durations. The relative strength and sensitivity of ON and OFF responses elicited by gaps varied with pulse rate. At 254 pps, OFF responses to the leading pulse train were evident down to gap durations as short as 4 ms, whereas ON bursts to the trailing pulse train were evident only for gaps 24 ms and longer. At 1,017 pps, however, it was the ON response to the trailing pulse train that persisted to the shortest gaps (as demonstrated by the latency of the spikes, measured relative to the onset of the trailing pulse train), whereas the response to the offset of the leading pulse train was evident only for gaps of 12 ms or longer. At the highest pulse rate (Figure 2C), there was no response to offset of the leading pulse train, but the unit responded reliably to the onsets of the pulse trains trailing the gaps.
ON and OFF responses were not simply superimposed, but showed nonlinear interactions. For instance, in the 254- and 1,017-pps examples in Figure 2, gap duration in the range of 8–24 ms resulted in an OFF response to the leading marker that collided with the ON response to the trailing marker, resulting in the appearance of a long-latency response to the trailing marker; that pattern was not seen in the 4,069-pps condition, in which there was no leading-marker OFF response. The appearance of a long-latency response after collision of OFF and ON responses was seen in several units in each of multiple animals and could be a rebound from inhibition enhanced by the coincidence of those responses. Figure 3 shows responses of the same unit in conditions of a shorter leading pulse train. In these early-gap conditions, there was little to no response to the offset of the leading pulse train. In contrast to the 254 pps long-lead case, ON responses were most informative about gap duration.
Gap detection thresholds vary with carrier pulse rate and gap position
As described in “Methods” section, the presence of gaps could be detected by the presence of responses to the offset of the leading pulse train (the Lead-OFF response), the onset of the trailing pulse train (the Trail-ON response), and/or a decrease in tonic activity during the silent gap (the TONIC response). Each of these features was observed for one or more units in each of six animals. We also observed suppression of spontaneous activity in response to electric stimulation in several units, but since these responses were evoked reliably in only two animals, we did not include an analysis in the current study. The apparent paucity of suppressive responses may be due to the difficulty of detecting such responses in the context of low spontaneous rate. To better understand the contributions of specific spike-train features to the overall pattern discrimination, we performed an ROC analysis of spike counts within time windows chosen to be selective for each feature. The most frequently observed feature was a response to the onset of the trailing pulse train consisting of a constellation of as many as three components: a temporally compact short-latency burst of spikes, a brief period of suppression, and a longer-latency elevation of spike rate. For most units, that Trail-ON response was present at the lowest current levels, at which no other stimulus-related feature could be detected. The latency of the initial burst tended to decrease with increasing stimulus level, reaching a median first-spike latency of 9.9 ms for stimuli at least 4.5 dB above threshold. All three components (short-latency ON, suppression, and long-latency ON) contributed to gap encoding. For instance, the long-latency response could signal the presence of a gap in conditions in which the short-latency ON response was not apparent. We computed the gap thresholds for each of the three components of the response to the trailing burst and reported the shortest as the Trail-ON threshold.
Responses to the offset of the leading pulse train (referred to as Lead-OFF) typically appeared at higher stimulus current levels than did Trail-ON responses, with a mean difference in thresholds of 3.7 dB for the 200 ms leading pulse train (p < 0.0001, Student’s t test). The median first-spike latency for Lead-OFF bursts, 19.9 ms, did not change systematically with level, and was longer than for ON responses for stimuli at least 4.5 dB above threshold (p < 0.0001, Kruskal–Wallis). OFF responses were preferentially evoked by longer stimulus durations, which can be seen in Figure 3A: the OFF response to the offset of the 225-ms uninterrupted pulse train in the 0-ms gap condition is more robust than the response to the offset of the 25-ms leading pulse train. Lead-OFF responses were more prevalent at lower pulse rates.
TONIC responses consisted of sustained firing throughout the duration of the stimulus. For some 25% of units, the presence of a gap in the stimulus could be detected by a significant decrease in tonic firing during the gap compared to the spike rate at a corresponding time in the standard no-gap stimulus. Decreases in tonic firing associated with gaps were susceptible to interference from OFF responses occurring within the gap.
The effect of pulse rate and gap placement on gap detection thresholds is summarized in Figure 4. The overall (“Best”; black bars) gap detection threshold for each unit was the shortest gap detection threshold derived from among the Trail-ON, Lead-OFF, and TONIC response features. The number of units that displayed sensitivity to gaps for one or more gap durations did not appear to vary systematically across stimulus conditions (Fig. 4A and B). Nevertheless, gap detection thresholds varied significantly across both pulse rate and gap position; statistical tests for specific conditions are given below. The distributions of gap thresholds given by specific features (Fig. 4C and D) reflect in each case only the units for which the particular feature was present; for instance, the distribution of Lead-OFF thresholds is based on the subset of units that showed a Lead-OFF response. For early gaps, median Best gap detection thresholds were 38, 26, and 12 ms for 254, 1,017, and 4,069 pps. For late gaps, median Best gap detection thresholds were 5.3, 1.4, and 0.77 ms, respectively. Best gap-detection thresholds were significantly shorter at higher pulse rates for both early and late gap conditions (p < 0.5, 1,017 vs. 4,069 pps, early gap; p < 0.001, all other comparisons; Kruskal–Wallis). The differences in median gap thresholds between early- and late-gap conditions were significant for all pulse rates (p < 0.001, Kruskal–Wallis) and were considerably larger than has been reported for normal-hearing human listeners (Phillips et al. 1998). Among the most sensitive units, however, the differences were only a few milliseconds. That is, the fifth percentiles for early- and late-gap thresholds were 6.6 and 2.3 ms, respectively, for 254 pps, 2.5 and 0.84 ms for 1,017 pps, and 1.9 and 0.25 ms for 4,069 pps.
Population summary of gap detection thresholds. The upper panels represent the numbers of units for which a gap detection threshold was obtained at any of the levels presented, for each analysis method and for the best gap detection threshold for each unit. The lower panels show the distributions of gap detection thresholds for each analysis, elicited by stimuli presented at least 4.5 dB above detection threshold. Filled bars represent the intraquartile range, circles represent the median gap detection thresholds, and tails indicate the 5th percentile. A, C Early-gap stimuli. B, D Late-gap stimuli.
The contribution of the various features of spike patterns to overall gap sensitivity varied with pulse rate and gap position. Figure 4C and D summarize gap detection thresholds obtained for each response type for gaps in pulse trains presented at least 4.5 dB above threshold to ensure that all three response features were represented. Statistical comparisons were made between groups using Kruskal–Wallis nonparametric analysis of variance. Across all units, Trail-ON responses were the most common responses to gaps, and gap thresholds obtained from Trail-ON responses were the shortest or were not significantly different from the Best gap detection thresholds for any stimulus condition (p = 0.05, 254-pps late gap; p > 0.40, all other pulse rate and gap position combinations). The 254 pps late gap condition showed a qualitative distinction in that a sizeable percentage of units (74%) exhibited a Lead-OFF response in that condition and that 70% of those units showed a shorter gap threshold for Lead-OFF responses than for Trail-ON response (50% of all units). Neither Lead-OFF nor Trail-ON responses were significantly more sensitive than the other across the population in the 254-pps condition (p = 0.83, Wilcoxon rank sum). In contrast, only 8% of all units showed a shorter gap threshold for Lead-OFF than for Trail-ON for the 1,017 pps late-gap condition and no units showed shorter Lead-OFF thresholds at 4,069 pps. Trail-ON responses were significantly more sensitive than Lead-OFF for these higher pulse rates (p < 0.00001). TONIC responses encoded both early and late gaps, but these gap detection thresholds were significantly less sensitive for all conditions (p < 0.001). In addition, there was no significant effect of gap position on TONIC-derived gap detection thresholds. In summary, Trail-ON responses were particularly important for gap sensitivity at high pulse rates and for early gaps, whereas Lead-OFF responses were at least as sensitive as Trail-ON responses for many units at 254 pps for late gaps. Each of these effects of gap position, stimulus pulse rate, and response feature were observed in each of the six animals.
Intracortical processing has been known to play a role in shaping sensitivity to temporal features in auditory stimuli (e.g., Eggermont 1999b; Middlebrooks 2008a). To test for a possible contribution of cortical columnar circuits to gap sensitivity, we aligned the data based on cortical depth relative to the input layers. Overall gap detection thresholds did not vary significantly with cortical depth (p > 0.05, Kruskal–Wallis for all stimuli; analysis limited to data presented at least 4.5 dB above threshold; data were grouped in 300-μm bins across cortical depth). We also compared the prevalence of each response type across cortical depth and found no significant localization of these response types. We infer that gap detection in a nonbehaving awake animal is largely inherited from subcortical auditory centers.
Gap detection thresholds depend on stimulus level
The results in Figure 4 showed a general decrease in gap detection thresholds associated with increasing pulse rate. Studies in human listeners, however, show that gap detection thresholds also decrease with increasing levels within a listener’s dynamic range (Garadat and Pfingst 2011) and that detection thresholds decrease at higher pulse rates (Kreft et al. 2004). For those reasons, there was a concern that our demonstration of a pulse-rate dependence of gap thresholds might have been confounded with a dependence on levels. In order to address that concern, we measured gap detection thresholds at multiple current levels. For each pulse rate in each animal, we tested three current levels relative to the modal detection threshold estimated online among jointly recorded units. Offline, we computed the detection threshold for each unit (as described in Methods) and expressed the three current levels relative to that unit’s threshold at that pulse rate. That procedure resulted in a continuum of levels tested relative to the thresholds in the unit sample. Moving averages of the gap detection thresholds obtained across these relative stimulus levels are plotted in Figure 5; top and bottom rows of panels represent early- and late-gap conditions, respectively. In each condition, sensitivity to gaps was poor for stimuli near threshold, and improved over the levels presented. Gap detection thresholds depended on stimulus level within the dynamic range for each stimulus condition. The range of stimulus levels over which gap detection thresholds varied with level appeared to be larger for 4,069 than 254 pps, which is consistent with reports of wider dynamic ranges at high pulse rates (Kreft et al. 2004). Gap detection thresholds shortened with increasing pulse rate in all six animals.
Gap detection thresholds by level. Each panel represents the distributions of gap detection threshold with level for each analysis type. Levels were calculated relative to the detection threshold for each unit. The shaded area denotes the intraquartile range and the line represents the median. Distributions were computed with averages taken across a moving window that encompassed 12.5% of the sample in each point, or a minimum of 13 points for small sample sizes. The upper panels A–C represent early gaps and the lower panels; C–E represent late gaps.
In human listeners, loudness matching reduces differences in gap detection thresholds across cochlear implant stimulation channels (Garadat and Pfingst 2011). However, the mechanisms for encoding “loudness” in neural data are not well established. We examined the dependence of gap detection thresholds on stimulus level to determine if there was a way to transform the resulting functions such that the difference in gap detection thresholds across pulse rate was minimized. Such a transformation could be considered a conservative estimate of “loudness matching”. However, it does not appear possible to transform the data in such a way. Detection thresholds generally were higher at lower pulse rates, so that the low-rate data were obtained at higher absolute current levels. For that reason, the differences in gap thresholds among pulse rates are even larger when expressed at absolute current levels than when expressed relative to detection thresholds (as in Figure 5). Furthermore, although detection thresholds and response growth were similar for early- and late-gap stimuli within each pulse rate, more sensitive responses were observed for late gaps at high stimulus levels. Therefore, we expect that the dependence of gap detection thresholds on pulse rate and gap position should withstand the equalizing effects of loudness matching in a psychophysical task. These observations of the sensitivity of gap thresholds demonstrate that, overall, differences in gap detection thresholds across pulse rate and gap position are not simply due to level effects.
Interactions between onset and offset responses to gaps
The relative importance of Trail-ON and Lead-OFF responses for encoding gaps in electric pulse trains varied with electric pulse rate. That is, in response to a 254-pps stimulus, the Lead-OFF response often produced a shorter gap threshold than did the Trail-ON response, and the opposite was observed at 1,017 and 4,069 pps pulse rates. We tested that rate-dependent switch in dominant feature across the range of stimulus levels. At 254 pps, only the Trail-ON response contributed a gap threshold at the lowest current levels, but as soon as levels increased to the point at which Lead-OFF responses were evident, many units showed a Lead-OFF response for shorter gaps than could be detected with Trail-ON responses (Figure 5D). At higher pulse rates, the Lead-OFF feature was absent at low stimulus levels, and for stimuli >4.5 dB above threshold, the Trail-ON feature generally yielded shorter gap thresholds, as shown in Figure 4. Thus, the Trail-ON feature was dominant at all stimulus levels for higher pulse rates.
We observed interactions between Lead-OFF and Trail-ON responses that seemed to indicate the presence of forward suppression by cortical neurons in which the leading response would suppress the lagging response. This appear to happen for the 254-pps stimulus in Figure 2A: the Trail-ON response is suppressed at times when it would occur less than 20 ms after the Lead-OFF response. If the cortical Lead-OFF response suppressed the Trail-ON response, however, similar suppression presumably would have occurred across all pulse rates. Contrary to that presumption, the Trail-ON response to the 1,017 pps stimulus in Figure 2B was not suppressed by the Lead-OFF response. In addition, Figure 2B demonstrated superimposed ON and OFF responses for gap durations at which the two responses were nearly simultaneous and demonstrated an absence of Lead-OFF responses for shorter gaps. Forward suppression is characterized by increasing amounts of suppression with decreasing interval, but in this case, the OFF response disappeared as the ON-OFF interval increased. This finding occurred throughout the population whenever the Trail-ON response preceded the Lead-OFF response window. Finally, Lead-OFF responses with long response latencies were blocked by Trail-ON responses to the gap. Although it is possible that forward suppression could last 100 ms, we routinely observed Trail-ON gap detection thresholds of much less than 75 ms, indicating that successive ON responses were not subject to inhibition in this fashion. For these reasons, the data do not support intracortical or thalamocortical forward suppression as the primary interaction between ON and OFF responses.
In a previous study in anesthetized guinea pigs with stimuli similar to the late-gap condition (Kirby and Middlebrooks 2010), no responses were observed to the offset of the leading marker, and yet the gap sensitivity of onset responses was very similar to Trail-ON gap detection thresholds in the present study. In the previous study, we found evidence for a retrocochlear forward suppression that was stronger at lower pulse rates, possibly because lower pulse rates produced less adaptation in auditory nerve input to brainstem sites (Zhang et al. 2007). Shorter leading pulse trains provide less time for adaptation to occur, and this difference should be greater at higher pulse rates. To test this hypothesis, we compared Trail-ON gap detection thresholds between early- and late-gap conditions in a subset of 32 units to which these stimulus pairs were presented consecutively. Trail-ON-derived gap detection thresholds were significantly longer for early gaps than late gaps for 1,017 pps (p < 0.01, Wilcoxon rank-sum; 32 units) and 4,069 pps (p < 0.01) but not for 254-pps (p = 0.82) stimuli. The log-ratio of the early- to the late-gap detection threshold was larger (i.e., longer gap detection thresholds at early gaps) for 4,069 than 254 pps (p < 0.0001, ANOVA) or 1,017 pps (p < 0.05), but there was no significant difference in the distributions obtained at 254 and 1,017 pps (p = 0.22). These results are consistent with increased adaptation in the auditory nerve at 4,069 pps leading to less forward suppression of ON-responses and thus more sensitive gap detection thresholds.
Lead-OFF responses were clearly dependent on gap duration and, by extension, on the onset of the trailing pulse train. In the present study, there were no examples of a Lead-OFF response occurring after a Trail-ON response. Therefore, at high pulse rates, the OFF-derived gap detection thresholds depended on the latency of the Lead-OFF response and the threshold of the Trail-ON response. Although the current data cannot tell us the level of the auditory pathway site at which responses to stimulus offset are generated, it appears that a preceding Trail-ON response has the ability to block the Lead-OFF response. Thus, the shortest Lead-OFF gap detection thresholds may be a byproduct of the forward suppression of Trail-ON responses.
Discussion
Recordings from the auditory cortex of the unanesthetized guinea pig demonstrated a diversity of features of spike patterns that could code temporal features of cochlear stimuli. The relative strengths of ON, OFF, and TONIC responses varied with stimulus conditions, including stimulus level, duration of the leading pulse train and, most conspicuously, the rates of electrical pulse trains. We begin our discussion by relating the present results to previous psychophysical and electrophysiological studies, including a discussion of sensitivity to electric pulse rates and to early and late gaps. We conclude with implications for cochlear implant listeners.
Relation to psychophysical studies
Gap detection studies in cochlear implant users have measured only thresholds for late-gap stimuli (i.e., leading markers ≥50 ms). At pulse rates ≤1,000 pps, gap detection thresholds were 1–5 ms for postlingually deafened subjects (Chatterjee et al. 1998; Shannon 1989) and 1–50 ms for prelingually deafened subjects, but there was no consistent relationship of gap thresholds with pulse rate. van Wieringen and Wouters (1999) observed gap detection thresholds of 2–8 ms at 400 pps and 1–4 ms at 1,250 pps, shorter at the higher pulse rate for three out of four listeners. In a test of very high pulse rates (3,000 pps), two users had gap detection thresholds of 1 ms or less (Grose and Buss 2007). Garadat et al. (2010) concluded that there was no consistent relationship between gap detection thresholds and pulse rate of 250, 1,000, and 4,000 pps across subjects. Nevertheless, they showed that gap detection thresholds were longer at the lowest pulse rate for five of six subjects: 5–15 ms for 4,000-pps and 15–75+ for 250-pps stimuli at the lowest current levels tested; and 1–5 ms for 4,000-pps and 4–12 ms for 250-pps stimuli at the highest current levels. For gap detection thresholds acquired in the middle of the dynamic range, where the greatest intersubject variability in gap detection thresholds arose, 250-pps stimuli were generally perceived as louder than 4,000-pps stimuli, a confounding factor. Overall, human psychophysical results display a trend of decreasing gap detection threshold with increasing pulse rate, but the effect of pulse rate is not as pronounced as in the present physiological study. Further studies that control for loudness might reduce variance among pulse rates and electrodes and thereby show a stronger systematic effect of pulse rate on gap detection threshold.
Early gaps have not been tested in cochlear implant listeners, but studies in normal-hearing listeners show little effect of gap position on gap detection threshold. Phillips et al. (1998) found a small (1–2 ms) but significant elevation of within-channel gap detection thresholds for early gaps in experienced listeners; that difference can be as high as 8 ms in inexperienced listeners (Snell and Hu 1999). The most sensitive 10% of units in the current study had gap detection thresholds of 2–8 ms across pulse rates for a 25-ms leading marker, indicating that the required sensitivity is available even in a passively hearing, naïve guinea pig.
Comparison with physiological studies
The current study is the first measurement of within-channel early-gap thresholds in awake animals. In a study in anesthetized cats (Eggermont 2000), thresholds for early gaps (i.e., 20-ms leading marker) were highly prolonged compared to late-gap physiological thresholds and to early-gap psychophysical thresholds (Phillips et al. 1998; Snell and Hu 1999). The prolonged early-gap thresholds observed under anesthesia likely are a result of the ∼50–200-ms period of suppression that typically follows the ON responses of cortical neurons in anesthetized conditions (e.g., Eggermont 2000; Kirby and Middlebrooks 2010). Prolonged early-gap thresholds were not seen in the present study in the absence of anesthesia.
Several studies have suggested that the presence of a gap in a stimulus could be signaled by a depression in ongoing neural activity. In the auditory nerve, for instance, gap-evoked fluctuations in sustained responses are particularly important for detection of the shortest gaps (Zhang et al. 1990). In addition, psychophysical studies of forward masking suggest that persistence of a response to a leading stimulus may contribute to masking of a subsequent probe (Plack and Oxenham 1998). Tonic responses are the most likely mediator of such an effect. This type of persistence was observed in a small proportion of neurons in the inferior colliculus of awake marmosets (Nelson et al. 2009) and in primary auditory cortex of guinea pigs (Alves-Pinto et al. 2010), but did not represent the limits of sensitivity to probes in either study. An effect similar to persistence was seen in the current study, as the sustained firing of TONIC responses continued for as long as 30 ms past stimulus offset, extending into the gap. Such persistent activity reduced the difference in firing rate between gap and no-gap conditions and limited TONIC-derived gap-detection thresholds to gaps longer than the duration of the persistent activity. Nevertheless, persistence and/or suppression of sustained activity had only a minor influence on detection of gaps in the present study. ON and OFF responses were more informative within the same responses and provided gap sensitivity more consistent with psychophysical data. The limits of physiological gap detection are not determined by the persistence of stimulus representation in the primary auditory cortex.
The current study confirms and extends our previous work in anesthetized guinea pigs (Kirby and Middlebrooks 2010). Gap detection thresholds obtained from Trail-ON responses were similar between ketamine-anesthetized and awake guinea pigs. However, only in awake animals did we see sizeable populations of units for which the Lead-OFF gap detection thresholds resulted in more sensitive gap detection thresholds for the 254-pps late-gap condition. Cortical OFF responses have been observed in spike activity of neurons in anesthetized cat (Eggermont 1999a) and in local field potentials in awake chinchilla (Guo and Burkard 2002) for leading markers 200 and 50 ms in duration, respectively, but only for gaps longer than 40 ms in duration. The most sensitive responses to gaps in noise bursts in these studies were always elicited by ON responses to trailing bursts. The high-temporal acuity Lead-OFF-dominant gap detection thresholds observed in the current study indicate that stimulation at 254 pps can in some cases produce temporal cues in the cortex unlike those elicited by acoustic stimulation.
Implications for cochlear implant users
The present results illustrate some features of cortical responses that could signal specific temporal elements of speech. Inasmuch as the prevalence of those cortical responses depends on electrical pulse rate, level, and duration of steady-state pulse trains (i.e., leading markers), those same stimulus parameters are likely to influence speech recognition. As an example, cross-channel gap detection thresholds for a 10-ms broadband noise leading marker and a 300-ms narrowband noise trailing marker were correlated with sensitivity to voice onset time in a synthesized ba-pa continuum in normal-hearing listeners (Elangovan and Stuart 2008), suggesting that performance in such a task is important for categorization of voiced and voiceless consonants. Lead-OFF responses such as those observed in the present study may be especially important in across-channel gap detection because cortical ON- and OFF- responses often are tuned to different frequencies in normal hearing (Qin et al. 2007), which could result in overlapping Lead-OFF and Trail-ON responses. In another study, gap duration discrimination performance within a synthesized-formant stimulus was correlated with performance on consonant and word discrimination tasks for cochlear implant listeners (Sagi et al. 2009). TONIC and Lead-OFF responses, which encode the duration of the leading marker, may provide a more relevant cue than the onset–onset interval.
We have shown that cortical responses to gaps vary qualitatively with carrier pulse rate. Higher pulse rates evoke more sensitive ON responses to gaps, and responses to the highest rate tested showed little representation of stimulus features other than pulse-train onsets. Low pulse rates, on the other hand, display robust coding of features throughout the duration of the stimulus, but do not drive ON responses to trailing markers at such short gap durations as those seen for higher pulse rates. Zhang et al. (2007) showed that the auditory nerve entrained to 250-pps pulse trains with little adaptation over the course of a 300-ms stimulus, whereas auditory nerve fibers showed significantly more spike rate adaptation at 5,000 pps. Based on the current results, a lack of adaptation over the course of a low pulse rate stimulus may result in enhanced representation of duration cues and enhanced forward suppression of subsequent stimuli in the central auditory system, whereas the sensitivity to successive stimulus onset shown for high-rate pulse trains comes at the cost of a degraded representation of the rest of the stimulus. We conclude that intermediate pulse rates, such as 1,017 pps, produce responses to gaps that are most similar to those seen in acoustic stimulation.
There is some evidence for the existence of a “sweet spot” in cochlear implant pulse rate. Amplitude modulation detection is impaired in human listeners at high pulse rates (2,000 or 4,000 pps), compared to low pulse rates (200 or 250 pps) (Galvin and Fu 2005; Pfingst et al. 2007); a similar result is found in auditory cortex of anesthetized guinea pigs (Middlebrooks 2008b). Arora et al. (2011) tested modulation detection threshold for carrier rates from 200 to 900 pps, and listeners were most sensitive for a carrier rate of 500 pps. A number of studies have measured the effect of pulse rate on speech performance, with mixed results. Recently, Battmer et al. (2010) performed a multistage, multicenter study and determined that performance was best at each listener’s preferred pulse rate, but 80% of listeners preferred pulse rates from 500 to 1,200 pps. The present results suggest that intermediate pulse rates elicit responses in the auditory pathway most similar to the responses to sound and, therefore, might be the most appropriate choice for many cochlear implant listeners.
References
Alves-Pinto A, Baudoux S, Palmer A, Sumner C (2010) Forward masking estimated by signal detection theory analysis of neuronal responses in primary auditory cortex. J Assoc Res Otolaryngol 11:477–494
Arora K, Vandali AE, Dowell R, Dawson P (2011) Effects of stimulation rate on modulation detection and speech recognition by cochlear implant users. Int J Audiol 50:123–132
Battmer R-D, Dillier N, Lai WK, Begall K, Leypon EE, González JCF, Manrique M, Morera C, Müller-Deile J, Wesarg T, Zarowski A, Killian MJ, von Wallenberg E, Smoorenburg GF (2010) Speech perception performance as a function of stimulus pulse rate and processing strategy preference for the Cochlear™ Nucleus® CI24RE device: relation to perceptual threshold and loudness comfort profiles. Int J Audiol 49:657–666
Chatterjee M, Fu QJ, Shannon RV (1998) Within-channel gap detection using dissimilar markers in cochlear implant listeners. J Acoust Soc Am 103:2515–2519
Eggermont JJ (1999a) Neural correlates of gap detection in three auditory cortical fields in the cat. J Neurophysiol 81:2570–2581
Eggermont JJ (1999b) The magnitude and phase of temporal modulation transfer functions in cat auditory cortex. J Neurosci 19:2780–2788
Eggermont JJ (2000) Neural responses in primary auditory cortex mimic psychophysical, across-frequency-channel, gap-detection thresholds. J Neurophysiol 84:1453–1463
Elangovan S, Stuart A (2008) Natural boundaries in gap detection are related to categorical perception of stop consonants. Ear Hear 29:761–774
Galvin JJ 3rd, Fu QJ (2005) Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J Assoc Res Otolaryngol 6:269–279
Garadat SN, Pfingst BE (2011) Relationship between gap detection thresholds and loudness in cochlear-implant users. Hear Res 275:130–138
Garadat SN, Thompson CS, Pfingst BE (2010) Gap detection for pulsatile electrical stimulation: effect of carrier rate and stimulus level. In: Midwinter Research Meeting of the Association for Research in Otolaryngology. Anaheim, CA, USA
Green DM, Swets JA (1966) Signal detection theory and psychophysics. Wiley, New York
Grose JH, Buss E (2007) Within- and across-channel gap detection in cochlear implant listeners. J Acoust Soc Am 122:3651–3658
Guo Y, Burkard R (2002) Onset and offset responses from inferior colliculus and auditory cortex to paired noisebursts: inner hair cell loss. Hear Res 171:158
Holden LK, Skinner MW, Holden TA, Demorest ME (2002) Effects of stimulation rate with the nucleus 24 ACE speech coding strategy. Ear Hear 23:463–476
Johnson MD, Otto KJ, Williams JC, Kipke DR (2004) Bias voltages at microelectrodes change neural interface properties in vivo. In: IEMBS, 26th Ann Int Conf IEEE, pp 4103–4106
Kirby AE, Middlebrooks JC (2010) Auditory temporal acuity probed with cochlear implant stimulation and cortical recording. J Neurophysiol 103:531–542
Kreft HA, Donaldson GS, Nelson DA (2004) Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users. J Acoust Soc Am 115:1885–1888
Liu Y, Qin L, Zhang X, Dong C, Sato Y (2010) Neural correlates of auditory temporal-interval discrimination in cats. Behav Brain Res 215:28–38
Loizou PC, Poroy O, Dorman M (2000) The effect of parametric variations of cochlear implant processors on speech understanding. J Acoust Soc Am 108:790–802
Macmillan NA, Creelman CD (2005) Detection theory: a user’s guide, 2nd edn. Lawrence Erlbaum Associates, Mahwah
Middlebrooks JC (2004) Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am 116:452–468
Middlebrooks JC (2008a) Auditory cortex phase locking to amplitude-modulated cochlear implant pulse trains. J Neurophysiol 100:76–91
Middlebrooks JC (2008b) Cochlear-implant high pulse rate and narrow electrode configuration impair transmission of temporal information to the auditory cortex. J Neurophysiol 100:92–107
Middlebrooks J, Snyder R (2007) Auditory prosthesis with a penetrating nerve array. J Assoc Res Otolaryngol 8:258
Muchnik C, Taitelbaum R, Tene S, Hildesheimer M (1994) Auditory temporal resolution and open speech recognition in cochlear implant recipients. Scand Audiol 23:105–109
Müller-Preuss P, Mitzdorf U (1984) Functional anatomy of the inferior colliculus and the auditory cortex: current source density analyses of click-evoked potentials. Hear Res 16:133
Nelson PC, Smith ZM, Young ED (2009) Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci 29:2553–2562
Nunamaker EA, Purcell EK, Kipke DR (2007) In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res A 83:1128–1137
Pfingst BE, Xu L, Thompson CS (2007) Effects of carrier pulse rate and stimulation site on modulation detection by subjects with cochlear implants. J Acoust Soc Am 121:2236–2246
Phillips DP, Hall SE, Harrington IA, Taylor TL (1998) “Central” auditory gap detection: a spatial case. J Acoust Soc Am 103:2064–2068
Plack CJ, Oxenham AJ (1998) Basilar-membrane nonlinearity and the growth of forward masking. J Acoust Soc Am 103:1598–1608
Plant K, Holden LK, Skinner MW, Arcaroli J, Whitford L, Law M-A, Nel E (2007) Clinical evaluation of higher stimulation rates in the nucleus research platform 8 system. Ear Hear 28:381–393
Qin L, Chimoto S, Sakai M, Wang J, Sato Y (2007) Comparison between offset and onset responses of primary auditory cortex ON-OFF neurons in awake cats. J Neurophysiol 97:3421–3431
Recanzone GH, Engle JR, Juarez-Salinas DL (2011) Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hear Res 271:115–122
Rubinstein JT, Wilson BS, Finley CC, Abbas PJ (1999) Pseudospontaneous activity: stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res 127:108
Rupp A, Gutschalk A, Uppenkamp S, Scherg M (2004) Middle latency auditory-evoked fields reflect psychoacoustic gap detection thresholds in human listeners. J Neurophysiol 92:2239–2247
Sagi E, Kaiser AR, Meyer TA, Svirsky MA (2009) The effect of temporal gap identification on speech perception by users of cochlear implants. J Speech Lang Hear Res 52:385–395
Shannon RV (1989) Detection of gaps in sinusoids and pulse trains by patients with cochlear implants. J Acoust Soc Am 85:2587–2592
Shannon RV, Cruz RJ, Galvin JJ (2011) Effect of stimulation rate on cochlear implant users’ phoneme, word and sentence recognition in quiet and in noise. Audiol Neurotol 16:113–123
Snell KB, Hu H-L (1999) The effect of temporal placement on gap detectability. J Acoust Soc Am 106:3571–3577
van Wieringen A, Wouters J (1999) Gap detection in single- and multiple-channel stimuli by LAURA cochlear implantees. J Acoust Soc Am 106:1925–1939
Verschuur CA (2005) Effect of stimulation rate on speech perception in adult users of the Med-El CIS speech processing strategy. Int J Audiol 44:58–63
Zeng FG, Kong YY, Michalewski HJ, Starr A (2005) Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol 93:3050–3063
Zhang W, Salvi RJ, Saunders SS (1990) Neural correlates of gap detection in auditory nerve fibers of the chinchilla. Hear Res 46:181–200
Zhang F, Miller CA, Robinson BK, Abbas PJ, Hu N (2007) Changes across time in spike rate and spike amplitude of auditory nerve fibers stimulated by electric pulse trains. J Assoc Res Otolaryngol 8:356–372
Acknowledgments
This work was supported by National Institute on Deafness and other Communication Disorders Grant R01 DC-04312. We thank C. Ellinger, C.-C. Lee, R. Adrian, L. Javier, S. Sameni, and E. McGuire for technical help with the experiments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kirby, A.E., Middlebrooks, J.C. Unanesthetized Auditory Cortex Exhibits Multiple Codes for Gaps in Cochlear Implant Pulse Trains. JARO 13, 67–80 (2012). https://doi.org/10.1007/s10162-011-0293-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-011-0293-0