Abstract
The hedgehog tenrec, Echinops telfairi, has certain basal mammalian features, like a cloaca and a sparsely differentiated brain with smooth cerebral hemispheres. The peripheral auditory capabilities of this species were investigated by means of distortion product otoacoustic emissions (DPOAE). For comparison, we determined auditory evoked potentials (AEP) in the inferior colliculus and the auditory cortex. Both methods show that the auditory range of E. telfairi extends well into ultrasonic frequencies, with a region of highest sensitivity at around 16 kHz. The total auditory range spans about 4 octaves at 40 dB SPL. The low-frequency limit of auditory processing is found at frequencies of about 2–3 kHz. The DPOAE and the AEP thresholds of E. telfairi do not run fully parallel in the high-frequency range. For a threshold value of 40 dB SPL, cochlear mechanical thresholds as measured with DPOAE extend up to 50 kHz, whereas neuronal thresholds reach the high-frequency limit at about 30 kHz. Frequency tuning, as assessed from DPOAE suppression tuning curves, was low to moderate with Q 10dB values ranging from 1.7 to 8. The lack of discontinuity in the group delay (derived from DPOAE measurements) reveals that cochlear frequency representation is tonotopic without any region of specialized mechanical tuning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Tenrecs are of the order insectivora, which is a rather ancestral group of eutherian mammals. It is argued that insectivores have retained primitive features and thus are closer to the ancestral stock than species of other living mammalian orders (see Springer et al. 1997), and that the study of insectivora brain organization provides insight into the brain of early ancestors of eutherian mammals (Stephan et al. 1991).
Besides advanced characteristics, tenrecs have retained conservative physiological and morphological features, e.g., an unstable thermoregulation and a reduced visual sense, the retention of testes within the body cavity, and a cloaca (Gould 1965; Eisenberg and Gould 1970). The encephalization index of tenrecs is the least among extant mammals, with a sizable bulbus olfactorius and smooth cerebral hemispheres (Stephan et al. 1991). The forebrain of tenrecs closely resembles that of early mammals in relative size and small neocortex (Künzle and Rehkämper 1992; Krubitzer et al. 1997). These features lead to the classification of tenrecs as primitive eutherian mammals.
Tenrecs are confined to Madagascar. They become active at dusk and hunt during the night. They search for insects on the ground and in the branches of trees and shrubbery (Eisenberg and Gould 1970). This lifestyle requires reliable orientation in the dark. Gould (1965) demonstrated the importance of hearing. Furthermore, he suggested that tenrecs are even capable of a crude mode of echolocation. They emit short clicks produced by tongue and lip movements, a technique similar to that utilized by the fruit bat Rousettus aegyptiacus, one of the few megachiropteran bats capable of echolocation (see Koay et al. 1998). These broadband calls, that cover a frequency range from about 5 to 17 kHz, may improve the near-field orientation of E. telfairi the night. Unfortunately, to our knowledge there exists no systematic study on the properties of the middle ear and the cochlea of this species.
We investigated the hearing capability of E. telfairi by determining the mechanical frequency processing of the inner ear by means of cochlear distortions. These frequency distortions are produced by a nonlinear amplification process of the inner ear (see, e.g., Dallos 1992) and are propagated back to the tympanum where they can be measured with a sensitive transducer. The distortion product otoacoustic emissions (DPOAE) have become a common tool in hearing research. They reflect general properties of a subject’s peripheral auditory processing (Probst et al. 1991; Whitehead et al. 1996; Shera et al. 1998) and can be used to predict auditory thresholds (Boege et al. 2002; Gorga et al. 1997). DPOAE-based auditory threshold curves usually correlate well with behavioral thresholds (Faulstich et al. 1996; Faulstich and Kössl 2000) We determined DPOAE thresholds of the relative sensitivity of nonlinear cochlear mechanics by presenting two pure tones of the primary frequencies f 1 and f 2 and measuring the most prominent cubic distortion product 2f 1−f 2. Mechanical tuning properties of the cochlea were derived from isosuppression tuning curves (STC).
Central processing of auditory information was investigated by recording auditory evoked potentials (AEP) in the inferior colliculus (IC) and in the auditory cortex (AC). Almost all auditory information converges in the midbrain auditory center, the IC, with extensive afferent projections to higher and lower auditory nuclei as well as to the motor system (Casseday and Covey 1996). It plays a direct or indirect role in virtually all sound-related behavior. The IC of different species has been the subject of many studies on central processing of auditory information and is well suited for an interspecies comparison. The neural thresholds of the AC give further information on central auditory processing. The investigation of the auditory capabilities of E. telfairi as a “basal” mammal should allow the assessment of evolutionary development of hearing in other eutheria and provide a basis for comparison with Monotremata and Marsupialia.
MATERIAL AND METHODS
A total of 19 adult lesser hedgehog tenrecs (E. telfairi) of both sexes were used in this study. The animals were taken from the breeding colony of H. Künzle (Künzle 1998). Animal weight varied from 70 to 185 g. This wide range of body weight is related to the seasonal activity pattern of E. telfairi, which undergoes a torpor period from about October to March in this colony. Anesthesia was initiated with Ethrane® (by inhalation). During experimentation, the animals were anesthetized with Tribromethanol (TBE, 1.0 ml/100 g BW, IP). The experiments were performed in a sound-attenuated chamber.
Otoacoustic emission measurements
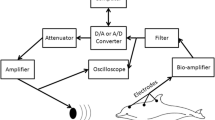
DPOAE measurements focused on 11 animals. Anesthesia was necessary to reduce animal movements and thus the risk of tympanic membrane injury caused by the acoustic coupler. A single recording session lasted up to 3 h. A homeothermic blanket was used to maintain the body temperature at about 29°C. The animal’s head was fixed with a mouth holder. A closed coupler system, containing three (0.5-in. B&K 4133 microphones that served as loudspeakers and one recording microphone (0.25-in. B&K 4135), was positioned in the animal’s meatus close to the eardrum. Two of the speakers were used to transmit the pure-tone stimuli (f 1 and f 2). The third optional speaker was used to present a suppressor tone (f 3) in the course of suppression tuning curve measurements (STC). A detailed description of the acoustic coupler system is given by Kössl (1994).
Stimulus generation and data acquisition were controlled by Testpoint programs (Keithley, Cleveland, OH). The pure-tone stimuli (f 1, f 2, and optionally f 3) were generated on two Microstar (Bellvue, WA) DAP digital signal-processing boards, D/A-converted, and fed to two or three, respectively, GPIB-controlled attenuators (designed by Jim Hartley, University of Sussex, Brighton), finally amplified and transmitted via the loudspeakers.
The speakers were separately calibrated in situ for frequencies between 1 and 100 kHz. If necessary, these acoustic measurements were repeated during the experiment (following animal head movements). The measured signals were amplified and fed into the A/D input of the DAP boards. The input and output channels of the board were sampled synchronously at 333 kHz per channel. The two primary stimuli f 1 and f 2 were presented at a starting phase of 0°. The starting phase of f 3 was not fixed.
Distortion threshold curves
DPOAEs are elicited by the interaction of two traveling waves on the basilar membrane induced by the simultaneous presentation of two pure-tone stimuli of different frequency (f 1 and f 2). In the zone of overlap both oscillations are amplified in a nonlinear way, thus producing frequency distortions. The place of distortion generation in the cochlea appears to be close to the frequency place of f 2 (f 1 < f 2; Brown and Kemp 1984). Consequently, we plotted the distortion threshold curve as a function of f 2. DPOAE threshold curves were measured for f 2 frequencies from 5 to 70 kHz. By keeping f 2 constant and varying f 1 the optimum stimulus separation (best ratio f 2/f 1) was determined, which produced maximum DPOAE levels at low stimulus levels. With the primary tones set at best ratio, growth functions of the 2f 1−f 2 distortion were measured by stepwise increasing the stimulus levels. The level of f 2 (l 2) was set to the level of f 1 (l 1) −10 dB, a stimulus condition proven to produce high DPOAE levels in mammals (Brown and Kemp 1984; Probst et al. 1991; Mills 1997). The level of f 2 sufficient to elicit a DPOAE of −10 dB SPL was defined as threshold criterion. Isodistortion threshold curves reflect auditory sensitivity in mammals as well as in reptiles (Kössl 1992, 1994; Manley et al. 1993; Faulstich et al. 1996; Faulstich and Kössl 1999).
DPOAE suppression tuning curves
Cochlear tuning properties were obtained by recording DPOAE suppression tuning curves (STC). After adjusting f 1 to the best ratio f 2/f 1, stimuli levels were chosen to elicit a 2f 1 − f 2 DPOAE between −5 to 5 dB SPL. At these levels, DPOAEs were more than 6 dB above the noise level and were sufficiently robust for suppression experiments. A pure-tone f 3, which was not phase fixed with respect to the stimuli, was presented simultaneously with the f 1 and f 2 stimuli via the third loudspeaker to suppress the DPOAE. For each primary stimulus pair, a large range of f 3 frequencies was tested. The f 3 level was increased in 5 dB steps and the DPOAE level was measured. The f 3 level sufficient to reduce the OAE by 6 dB was plotted as a function of the f 3 frequency to derive the tuning curve (e.g., Brown and Kemp 1984; Martin et al. 1987). The separation of f 3 from the stimulus frequencies and to the DPOAE frequency was at least 200 Hz to avoid direct interaction of the tones. From the STC the best frequency (BF), i.e., the frequency of the absolute minimum of the STC, the threshold at BF, and the Q 10dB were determined.
DPOAE group delay
Evoked otoacoustic emissions have a certain latency relative to the onset of the stimuli. In the case of DPOAEs, this latency is thought to depend on the cochlear traveling times and on the delay of the distortion generation. It is difficult to determine the latency from measurements in the time domain since stimuli and DPOAEs largely overlap. However, the phase of the DPOAE can be used to measure the ongoing transmission delay of DPOAEs. Specifically, the gradient of DPOAE phase (ph) versus DPOAE frequency (f) defines the group delay, which is the ongoing delay of the main emission wave front (e.g., Kimberley et al. 1993). The magnitude of the group delay of DPOAEs is a measure of the travel time and depends on the stimulus paradigm used (O’Mahoney and Kemp 1995; Whitehead et al. 1996). To gain information on cochlear traveling times and under the assumption that the 2f 1−f 2 distortion is generated at the f 2 representation site (e.g., Brown and Kemp 1984), the frequency of f 2 was fixed and f 1 was shifted to lower frequencies. The corresponding level and the phase of the 2f 1 − f 2 DPOAE were measured for different f 2 frequencies and at a number of stimulus levels. The measured phase of the DPOAE was corrected for the stimulus phases according to Mills and Rubel (1997). After unrolling phase transitions from –180° to +180° by subtracting 360°, the mean group delay (−Δph/Δf * 360°) was calculated over the frequency range for which the DPOAE level reached its maximum. The group delay values were plotted as a function of f 2.
Neurophysiological recording
We recorded AEPs of E. telfairi in eight specimens over a stimulus frequency range of 2–80 kHz. For surgery, the animal’s head was stabilized with a mouth holder and two ear pins. The pedal withdrawal reflex served as a measure for the state of anesthesia. An incision along the midsaggital plane of the skull was made and the tissue was pushed laterally with a retractor. A metal pin was glued to the midline of the skull with dental cement. This pin served to attach the animal to the stereotactic device (see Schuller et al. 1986). When the animal was placed in the experimental setup, its position was aligned along three axes to achieve a standard position. A reference point on the surface of the skull (hemispheric divergence, hd) was defined. The penetration point was located relative to this mark. For each electrode penetration, a small hole was drilled into the skull.
Tungsten electrodes were used for AEP recording (impedance <2 MΩ). The signal was amplified [Bioamp Headstage HS4; Tucker Davis Technologies (TDT), Gainesville, FL, Bioamp controller DB4], band pass filtered (TDT Bioamp controller DB4; Spike Conditioner PC1), monitored on an oscilloscope (Hameg HM 1007), and fed via a custom-made interface to a PC for analysis. Usually 20 passes per stimulus presentation were averaged.
Pure-tone stimuli were used to determine the neural response. The presentation interval was 500 ms and the stimulus duration was 200 ms. The rise/fall time was set to 3 ms. The stimuli were generated on an array processor card (TDT AP2), D/A-converted (TDT DA3-2), and fed to an anti−aliasing filter (TDT FT5). The stimulus level was set with two programmable attenuators (TDT PA4) connected in series. The stimuli were amplified (Rotel Power Amplifier RB 951, Rotel, North Reading, MA) and transmitted to the calibrated loudspeaker, which was positioned at a distance of 25 cm, aimed to the center of the pinna at an angle of 45° contralateral to the neurophysiological recording side. The sound system was calibrated in the recording setup, where a 0.25-in. B&K microphone was placed instead of the animal at the position of the tympanum to record the frequency response of the loudspeakers. The frequency response of the used speaker was flat within ±10 dB for frequencies between 10 to 80 kHz. Harmonic distortions of the speaker for the stimulus frequencies used were at least 40 dB below the level of the pure-tone stimulus and therefore did not produce artefacts. Stimulus presentation, signal recording, and averaging were controlled by a timing generator (TDT TG6). The threshold of the AEP was determined as that stimulation level at which the averaged neuronal response was no longer distinguishable from the noise floor. The level resolution was set to 5 dB.
RESULTS
Distortion threshold curves
The distortion thresholds were determined for f 2 frequencies from 5 to 70 kHz (Fig. 1). The best frequency ratios (f 2/f 1) resulting in highest levels of 2f 1 − f 2 ranged from 1.11 to 1.29. No significant correlation between the best frequency ratio and f 2 could be found. The threshold curve of E. telfairi is broadly tuned with a shallow low-frequency and a steeper high-frequency flank. It ranges from about 5 to 50 kHz (at 40 dB SPL). Below 5 kHz no DPOAE could be elicited, even with primary f 2 levels of 70 dB SPL. The minimum of the average distortion threshold was between 10 and 30 kHz at f 2 levels of approximately 20 dB SPL. The large variability of the threshold data may partly be caused by physiological changes that are linked to variations of general activity of the animal.
DPOAE threshold curve from 5 to 70 kHz for E. telfairi. The level of f 2 (l 2; l 2 = l 1 − 10 dB) sufficient to generate a 2f 1 − f 2 DPOAE of −10 dB SPL is plotted as a function of the f 2frequency. The best frequency ratios (f 2/f 1) resulting in highest levels of 2f 1 − f 2 ranged from 1.11 to 1.29. No significant correlation between the best frequency ratio and f 2 could be found. Means (filled circles) and standard deviations (error bars) are shown.
Suppression tuning curves
The isosuppression tuning curves (STC) measured in the tenrec display an almost symmetrical shape with the low-frequency flank only slightly shallower than the high-frequency flank. Frequency tuning sharpness was low to moderate with Q 10dB values ranging from 1.7 to 8. The minimum was close to f 2 for all STCs at f 2 < 30 kHz (Fig. 2), suggesting that the generation of DPOAE takes place near the f 2 place on the basilar membrane like in other mammals (e.g., Brown and Kemp 1984). For f 2 > 30 kHz, the minimum of the STCs determined in one individual did not correlate with f 2 anymore but stayed around 30–35 kHz (Fig. 2f). This was also found in the opossum for f 2 frequencies around 45 kHz (Faulstich et al. 1996). The best frequency ratios (f 2/f 1) resulting in highest levels of 2f 1 − f 2 ranged from 1.11 to 1.33 in this animal. There was no correlation between the best frequency ratio and the Q 10dB value.
DPOAE isosuppression tuning curves from one animal. The level of f 3 necessary to suppress 2f 1 − f 2 by 6 dB is plotted as a function of the frequency of f 3. Circles represent the frequency and level of the primaries. The STC’s Q 10dB values are shown in the lower left of each panel. For f 2 frequencies greater than 30 kHz, the minimum of the STCs no longer follows the primary frequencies. The f 2 frequency is indicated at the top of the corresponding panel. The best frequency ratios (f 2/f 1) resulting in highest levels of 2f 1 −f 2 ranged from 1.11 to 1.33. There was no correlation between the best frequency ratio and the Q 10dB value. The level of f 2 was set to the level of f 1 – 10 dB.
Group delay
When f 2 is held constant and f 1 is lowered in small steps, 2f 1 − f 2 DPOAEs can be measured for a wide range of f 1 frequencies. The DPOAE phase changes regularly with each f 1 step and a rather constant group delay value results, indicating the slope of the phase gradient. The group delay was averaged over as many points as possible (at least 15 points) for which a continuous phase was present (for a detailed methodological description, see Faulstich and Kössl 2000).
The group delay was measured in a single individual of E. telfairi where large distortions could be elicited over a wide frequency range. The stimulus level was at least 6 dB above distortion threshold levels. The mean group delay decreased with increasing f 2. It was highest (2.23 ms) for f 2 = 5 kHz and drops to a minimum of 0.17 ms for f 2 = 40 kHz. The course of the mean group delay is similar to those measured in the gerbil and the opossum with the same experimental setup (Fig. 3).
The group delay (ms) of the distortion product as a function of frequency (kHz) of the tenrec (solid line). For comparison, data from the gerbil (dashed line) and the opossum (dotted line) are shown. To determine the group delay, f 2 was held constant while f 1 was decreased in small steps. For each f 1 step, the 2f 1 − f 2 DPOAE level and its phase was determined as a function of the 2f 1 − f 2 frequency. Fromthe change in DPOAE phase relative to the change in frequency, the mean group delay values were calculated over all values for which the ratio curve was at least 6 dB above noise floor.
Neurophysiological thresholds
Auditory evoked potentials from a total of 22 electrode penetrations in eight animals were analyzed to determine the neurophysiological threshold of E. telfairi in the IC. In the presumed AC, 24 electrode penetrations in six animals were conducted. Frequencies from 2 to 80 kHz were tested comprising E. telfairi’s hearing range as determined in the preceding otoacoustic experiments. The penetration loci in the IC were distributed over the whole nucleus including rostral, central, caudal, and lateral parts (see Fig. 4a) at different penetration depths (covering the whole dorsoventral extension of the IC of 3000 µm). The shape as well as the absolute sensitivity of individual EP thresholds was rather similar across penetrations, indicating that the electrodes used did not locally discriminate and yielded a gross response covering the entire IC (Fig. 4b). Control measurements adjacent to the IC did not elicit responses to auditory stimuli.
Evoked potential recording in the IC. a IC–recording positions. The penetration sites across individuals were normalized according to two references; Cbl−Cerebellum, IC−Colliculus inferior, NCx−Neocortex, SC−Colliculus superior, hd−hemispheric divergence. b Examples of audiograms from separated loci across the IC in one animal. Regardless of the penetration site, the course of the threshold curves evoked by acoustic stimulation is similar.
Similar to the threshold determined by DPOAE measurements, the neurophysiological response extends to ultrasonic frequencies (Fig. 5). The range of best sensitivity both in the IC and the AC is found between 8 and 20 kHz with a threshold minimum of about 10 dB SPL (IC) or 5 dB SPL (AC), respectively, at 16 kHz. In comparison to the DPOAE data, the threshold curve of AEP is slightly shifted to lower frequencies. The lower−frequency range measured with AEPs extends to 2–3 kHz in comparison to 5 kHz for the DPOAE thresholds. For frequencies above 20 kHz, the neurophysiological threshold consistently increases, reaching the upper limit at about 30 kHz (at 40 dB SPL). Thus, IC and AC thresholds are tuned to lower frequencies and the distortion product threshold is more broadly tuned than the neurophysiological response.
DISCUSSION
The auditory range of E. telfairi extends to ultrasonic frequencies with a broad threshold minimum. The frequency range of this region differs, depending on whether the ascending auditory pathway was investigated at a peripheral or a central position. The DPOAE threshold curve is plotted as a function of f 2 frequency since the cubic distortions products, which provide the basis for the threshold curves, are generated close to the f 2 frequency place (e.g., Brown and Kemp 1984). It reveals a frequency range of minimum thresholds between 10 and 35 kHz (Fig. 1). The associated f 2 level sufficient to elicit distortions is slightly below 20 dB SPL. The threshold ranges from about 5 to 50 kHz (at 40 dB SPL).
The thresholds based on AEPs, recorded in the IC and the AC, reveal a low-frequency limit of 2–3 kHz. At frequencies above 25 kHz, the neuronal auditory sensitivity decreases steeply with increasing frequency (Fig. 6). The differences among otoacoustic and neurophysiological thresholds at low frequencies are also found in other mammals, e.g., in the gerbil Meriones unguiculatus (Faulstich and Kössl 2000), in the rhesus monkey Macaca mulatta (Lasky et al. 1999), in the Echidna (Tachyglossus aculeatus; Mills and Shepherd 2001), and in the opposum (Monodelphis domestica; Faulstich et al. 1996). In these species the course of neurophysiologically measured threshold curves extends to lower frequencies than that of DPOAE thresholds (see also Fig. 7). In general, differences between DPOAE measurements made with a closed system and free-field neurophysiological data might be caused by a notch in the head related transfer function (HRTF) or an antiresonance in the free-field system. Nevertheless, neurophysiological and DPOAE data from the Echidna (Fig. 7) were both obtained under closed-field conditions, but still reveal differences comparable to the ones found in the tenrec, suggesting that the arguments given above might contribute to these differences, but may not be the sole source. Differences between DPOAE and neurophysiological data seem to be a general feature found in a wide range of mammals and suggest an intrinsic difference between neuronal and otoacoustic emission responses, which might be related to the suggestion that DPOAEs reflect the activity of the cochlear amplifier, whereas neuronal threshold curves are further shaped by additional processes in the ascending auditory pathway. When assessing the difference between DPOAE and neurophysiological data, one needs to keep in mind that the middle ear has more influence on the DPOAE than on neurophysiological measures. In the gerbil, it may act as a high-pass filter, thus altering the measurable distortion product in the ear canal (Faulstich and Kössl 1999). However, since E. telfairi is much less sensitive for low frequencies than the gerbil, middle-ear high-pass filtering seems to be less important. The neurophysiological range is shifted toward lower frequencies with an area of highest sensitivity between 10 and 16 kHz. In addition to the suggestion made above, this might also be related to the auditory gain originating from the pinna, since it is known that in other species, depending on the size of the pinna, amplification effects are most prominent at frequencies ranging from about 2.5 to 30 kHz (e.g., Murphy and Davis 1998; Guppy and Coles 1988; Coles and Guppy 1986; Jen and Chen 1988) and can amount to values as much as 20 dB. As the DPOAEs were measured directly at the eardrum, pinna effects are excluded in these measurements. The neurophysiological data reveals an upper limit of about 30 kHz (at 40 dB SPL), associated with a sharp high-frequency cutoff, corresponding to more than 30 dB/octave. Despite the relatively high standard deviations, the AEP high-frequency threshold is up to 30 dB above that of the concomitant DPOAE threshold.
The peripheral tuning, determined by isosuppression tuning curves (STC), displays an almost symmetrical shape. Q 10dB values of 1.7–8 reveal a low to moderate frequency tuning (Fig. 2). The minimum was close to f 2 for frequencies below 30 kHz. Consequently, like in other mammals, the distortion generation most probably took place near the f 2 position. For frequencies above 30 kHz, the minimum in STC was not correlated to f 2 but stayed around 30–35 kHz (Fig. 2f). The same response characteristics were also found in the opossum Monodelphis domestica at f 2 frequencies around 45 kHz and may indicate the end of frequency representation in the cochlea (Faulstich et al. 1996), which is further supported by the steep increase of the distortion threshold at high frequencies.
Evoked otoacoustic emissions have a certain latency relative to the onset of the stimuli. In the case of DPOAEs, this latency is thought to depend on the cochlear traveling times and on the delay of the distortion generation. Thus, the calculation of the group delay, expressed as the gradient of DPOAE phase versus DPOAE frequency, can give information about the peripheral frequency representation and specializations in cochlear mechanics.
The mean group delay of E. telfairi decreases with increasing f 2 from 2.23 ms at 5 kHz to 0.17 ms at 40 kHz (Fig. 3). The course of the mean group delay (Fig. 3) is similar to those of the gerbil (Faulstich and Kössl 2000) and the opossum. The minimum values are almost identical for the three species for f 2 frequencies greater than 10 kHz. For lower frequencies, the mean group delay reaches similar values for different frequencies and seems to be correlated to the lower flank of the threshold. The lack of discontinuity in the group delay reveals that the peripheral frequency representation is tonotopic without any region of enhanced mechanical tuning.
Both the range and the shape of the threshold of E. telfairi correspond well to the typical mammalian pattern. Nevertheless, the comparison within eutherian mammals reveals differences in low-frequency auditory processing. When investigating the low-frequency limit of 58 mammalian species, Heffner et al. (2001) found a bimodal distribution with species sensitive to frequencies below 125 Hz being separated from those with a low-frequency limit above 500 Hz. Nevertheless, there is no obvious morphologic, phylogenetic or ecological parameter that allows a distinction between members of these two groups. A similar divergence is found in rodents. Restricted low-frequency processing is found, for example, in the spiny mouse (Acomys cahirinus), whereas the chipmunk (Tamias striatus), the groundhog (Marmota monax), the hamster (Mesocricetus auritus), the chinchilla (Chinchilla chinchilla), and the gerbil (Meriones unguiculatis) belong to the “low-frequency” group (Heffner and Heffner 1991; Faulstich and Kössl 1999; Heffner et al. 2001). The low-frequency limit apparently depends on features that are not primarily related to a species phylogeny. Anatomical investigations of the cochlea indicate that, concerning its acoustic sense, E. telfairi is a generalist without any obvious specializations (M. Vater, personal communication).
When compared with eutherians, marsupials are still considered “primitive,” although they successfully adapted to a wide range of different lifestyles. These adaptations certainly include the auditory system. The middle ear and the inner ear as well as the neuronal organization and connectivity were studied in representatives and found to be rather similar to those of eutherians (Willard and Martin 1983; Muller et al. 1993; Aitkin 1995). The hearing capabilities of the echidna, Tachyglossus aculaetus (Monotremata), are found to be midway between mammals and birds (Mills and Shepherd 2001). This species resembles E. telfairi in both appearance and lifestyle. Tachyglosssus’ internal temperature is relatively low (<32°C) for mammals, and it possesses a cloaca. Its acoustic sensitivity, investigated by DPOAE and auditory brainstem responses, is best in a frequency range of 4–8 kHz and the absolute threshold values are comparable to that of eutherian mammals (Fig. 7). However, the effective auditory range comprises only 3 octaves, and the high-frequency limit of T. aculaetus is restricted to frequencies unusually low for mammals. The auditory range of E. telfairi with a high-frequency limit of 40 kHz and a frequency range of only 4 octaves resembles that of T. aculaetus. Mammals typically are able to perceive frequencies up to 30–50 kHz. Specialists extend this range to beyond 100 kHz. Even in birds with a specialized auditory system, the hearing range is restricted to frequencies below 12 kHz (for review, see Manley 1990).
Variations in high-frequency hearing have been attributed to the evolutionary pressure involving sound localization, as a species’ high-frequency hearing limit varies inversely with interaural distance. Mammals with small heads and ears need to hear higher frequencies to use interaural intensity differences and pinna cues for sound localization than larger mammals (Heffner et al. 2001). Early mammals are supposed to have been insectivore-like, with small heads and a good high-frequency hearing (Popper and Fay 1997). Apparently, E. telfairi, with an auditory threshold comprising about 4 octaves, including restricted low-frequency hearing, a high-frequency limit at about 40 kHz, and a moderate sensitivity in the range of best hearing between about 10 and 16 kHz, complies with eutherian ancestors.
References
LM Aitkin (1995) ArticleTitleThe auditory neurobiology of marsupials: a review. Hear. Res. 82 257–266 Occurrence Handle10.1016/0378-5955(94)00182-P Occurrence Handle1:STN:280:ByqB1MzotVQ%3D Occurrence Handle7775290
P Boege T Jansen (2002) ArticleTitlePure-tone threshold estimation from extrapolated distortion product otoacoustic emission I/O-functions in normal and cochlear hearing loss ears. J. Acoust. Soc. Am. 111 1810–1818 Occurrence Handle10.1121/1.1460923 Occurrence Handle12002865
AM Brown DT Kemp (1984) ArticleTitleSuppressibility of the 2f l−f 2 stimulated acoustic emissions in gerbil and man. Hear. Res. 13 29–37 Occurrence Handle10.1016/0378-5955(84)90092-3 Occurrence Handle1:STN:280:BiuC287msFA%3D Occurrence Handle6706860
JH Casseday E Covey (1996) ArticleTitleA neuroethological theory of the operation of the inferior colliculus. Brain Behav. Evol. 47 311–336 Occurrence Handle1:STN:280:BymA1cboslQ%3D Occurrence Handle8796964
RB Coles A Guppy (1986) ArticleTitleBiophysical aspects of directional hearing in the tammar wallaby, Macropus eugenii. J. Exp. Biol. 121 371–394
P Dallos (1992) ArticleTitleThe active cochlea. J. Neurosci. 12 4575–4585 Occurrence Handle1:STN:280:ByyC3c%2FlsVM%3D Occurrence Handle1464757
JF Eisenberg E Gould (1970) ArticleTitleThe tenrecs: A study in mammalian behavior and evolution. Smiths. Contrib. Zool. 27 1–138
M Faulstich M Kössl (1999) ArticleTitleNeuronal response to cochlear distortion products in the anteroventral cochlear nucleus of the gerbil. J. Acoust. Soc. Am. 105 491–502 Occurrence Handle10.1121/1.424586 Occurrence Handle1:STN:280:DyaK1M7itVansw%3D%3D Occurrence Handle9921673
M Faulstich M Kössl (2000) ArticleTitleEvidence for multiple DPOAE components based upon group delay of the 2f(1)−f(2) distortion in the gerbil. Hear. Res. 140 99–110 Occurrence Handle10.1016/S0378-5955(99)00189-6 Occurrence Handle1:STN:280:DC%2BD3c7ktFegsg%3D%3D Occurrence Handle10675638
M Faulstich M Kössl K Reimer (1996) ArticleTitleAnalysis of non-linear cochlear mechanics in the marsupial Monodelphis domestica: ancestral and modern mammalian features. Hear. Res. 94 47–53 Occurrence Handle10.1016/0378-5955(95)00232-4 Occurrence Handle1:STN:280:BymA1c7nvFM%3D Occurrence Handle8789810
MP Gorga ST Neely B Ohlrich B Hoover J Redner J Peters (1997) ArticleTitleFrom laboratory to clinic: a large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 18 440–455 Occurrence Handle1:STN:280:DyaK1c%2FnvVCjtQ%3D%3D Occurrence Handle9416447
E Gould (1965) ArticleTitleEvidence for echolocation in the Tenrecidae of Madagascar. Proc. Am. Phil. Soc. 109 352–360
A Guppy RB Coles (1988) ArticleTitleAcoustical and neuronal aspects of hearing in the Australian gleaning bats, Macroderma gigas and Nyctophilus gouldi. J. Comp. Physiol. A 162 653–668 Occurrence Handle1:STN:280:BieB3s%2FivVI%3D Occurrence Handle3373455
RS Heffner HE Heffner (1991) ArticleTitleBehavioral hearing range of the chinchilla. Hear. Res. 52 13–16 Occurrence Handle10.1016/0378-5955(91)90183-A Occurrence Handle1:STN:280:By6B1cngs1E%3D Occurrence Handle2061202
RS Heffner G Koay HE Heffner (2001) ArticleTitleAudiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hear. Res. 157 138–152 Occurrence Handle10.1016/S0378-5955(01)00298-2 Occurrence Handle1:STN:280:DC%2BD3MvitFWguw%3D%3D Occurrence Handle11470193
H-S Jen D Chen (1988) ArticleTitleDirectionality of sound pressure transformation at the pinna of echolocating bats. Hear. Res. 34 101–118 Occurrence Handle10.1016/0378-5955(88)90098-6 Occurrence Handle1:STN:280:BiaD3cjot1A%3D Occurrence Handle3170353
BP Kimberley DK Brown JJ Eggermont (1993) ArticleTitleMeasuring human cochlear traveling wave delay using distortion product emission phase responses. J. Acoust. Soc. Am. 94 1343–1350 Occurrence Handle1:STN:280:ByuD3Mzos1I%3D Occurrence Handle8408975
G Koay RS Heffner HE Heffner (1998) ArticleTitleHearing in a megachiropteran fruit bat (Rousettus aegyptiacus). J. Comp. Psychol. 112 371–382 Occurrence Handle10.1037//0735-7036.112.4.371 Occurrence Handle1:STN:280:DyaK1M%2FnvVOjsQ%3D%3D Occurrence Handle9861710
M Kössl (1992) ArticleTitleHigh-frequency distortions from the ears of two bat species, Megaderma lyra and Carollia perspicillata. Hear. Res. 60 IssueID2 156–164 Occurrence Handle10.1016/0378-5955(92)90018-I Occurrence Handle1639726
M Kössl (1994) ArticleTitleOtoacoustic emissions from the cochlea of the “constant frequency” bats, Pteronotus parnellii and Rhinolophus rouxi. Hear. Res. 72 59–72 Occurrence Handle10.1016/0378-5955(94)90206-2 Occurrence Handle8150746
L Krubitzer H Künzle JH Kaas (1997) ArticleTitleOrganization of sensory cortex in a Madagascan insectivore, the tenrec (Echinops telfairi). J. Comp. Neurol. 379 399–414 Occurrence Handle10.1002/(SICI)1096-9861(19970317)379:3<399::AID-CNE6>3.3.CO;2-3 Occurrence Handle1:STN:280:ByiB38%2FmslQ%3D Occurrence Handle9067832
H Künzle (1998) ArticleTitleCare and breeding of the Madagascan hedgehog tenrec, Echinops telfairi, under laboratory conditions. Der Tierschutzbeauftragte 7 113–115
H Künzle G Rehkämper (1992) ArticleTitleDistribution of cortical neurons projecting to dorsal column nuclear complex and spinal cord in the hedgehog tenrec, Echinops telfairi. Somatosens. Mot. Res. 9 185–197 Occurrence Handle1414117
RE Lasky AA Soto ML Luck NK Laughlin (1999) ArticleTitleOtoacoustic emission, evoked potential, and behavioral auditory thresholds in the rhesus monkey (Macaco mulatta). Hear. Res. 136 35–43 Occurrence Handle10.1016/S0378-5955(99)00100-8 Occurrence Handle1:STN:280:DyaK1Mvkt1Cjug%3D%3D Occurrence Handle10511622
GA Manley (1990) Peripheral hearing mechanisms in reptiles and birds. Springer Verlag Berlin 288
GA Manley C Köppl BM Johnston (1993) ArticleTitleDistortion-product Otoacoustic emissions in the bobtail lizard. I: General characteristics. J. Acoust. Soc. Am. 93 2820–2833
GK Martin BL Lonsbury–Martin R Probst SA Scheinin AC Coats (1987) ArticleTitleAcoustic distortion products in rabbit ear canal. II. Sites of origin revealed by suppression contours and pure−tone exposures. Hear. Res. 28 191–208 Occurrence Handle10.1016/0378-5955(87)90049-9 Occurrence Handle1:STN:280:BieD3cjgvV0%3D Occurrence Handle3654389
DM Mills (1997) ArticleTitleInterpretation of distortion product Otoacoustic emission measurements. I. Two stimulus tones. J. Acoust. Soc. Am. 102 413–429 Occurrence Handle10.1121/1.419763 Occurrence Handle1:STN:280:ByiA2s3lvVA%3D Occurrence Handle9228804
DM Mills EW Rubel (1997) ArticleTitleDevelopment of distortion product emissions in the gerbil: “filter” response and signal delay. J. Acoust. Soc. Am. 101 395–411 Occurrence Handle10.1121/1.417985 Occurrence Handle1:STN:280:ByiC2MzotVU%3D Occurrence Handle9000732
DM Mills RK Shepherd (2001) ArticleTitleDistortion product otoacoustic emission and auditory brainstem responses in the echidna (Tachyglossus aculeatus). J. Assoc. Res. Otolaryngol. 2 130–146
M Muller (1996) ArticleTitleThe cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear. Res 94 148–156 Occurrence Handle10.1016/0378-5955(95)00230-8 Occurrence Handle1:STN:280:BymA1c7otVM%3D Occurrence Handle8789820
M Muller FP Wess V Bruns (1993) ArticleTitleCochlear place-frequency map in the marsupial Monodelphis domestica. Hear. Res. 67 198–202 Occurrence Handle10.1016/0378-5955(93)90247-X Occurrence Handle1:STN:280:ByyA3sbpvVU%3D Occurrence Handle8340271
WJ Murphy RR Davis (1998) ArticleTitleThe role of the chinchilla pinna and ear canal in electrophysiological measures of hearing thresholds. J. Acoust. Soc. Am. 103 1951–1956 Occurrence Handle10.1121/1.421376 Occurrence Handle1:STN:280:DyaK1c3islCntA%3D%3D Occurrence Handle9566318
CF O’Mahoney DT Kemp (1995) ArticleTitleDistortion product otoacoustic emission delay measurement in human ears. J. Acoust. Soc. Am. 97 3721–3735 Occurrence Handle7790651
AN Popper RR Fay (1997) ArticleTitleEvolution of the ear and hearing: issues and questions. Brain Behav. Evol. 50 213–221 Occurrence Handle1:STN:280:ByiH2MrpvFc%3D Occurrence Handle9310196
R Probst BL Lonsbury−Martin GK Martin (1991) ArticleTitleA review of otoacoustic emissions. J. Acoust. Soc. Am. 89 2027–2067 Occurrence Handle1:STN:280:By6A3MfgsVI%3D Occurrence Handle1860995
K Reimer (1995) ArticleTitleHearing in the marsupial Monodelphis domestica as determined by auditory-evoked brainstem responses. Audiology 34 334–342 Occurrence Handle1:STN:280:BymH3s%2FhvVE%3D Occurrence Handle8833313
AF Ryan (1976) ArticleTitleHearing sensitivity of the mongolian gerbil, Meriones unguiculatis. J. Acoust. Soc. Am. 59 1222–1226 Occurrence Handle1:STN:280:CSmB28%2Fot1A%3D Occurrence Handle956517
G Schuller S Radtke–Schuller M Betz (1986) ArticleTitleA stereotaxic method for small animals using experimentally determined reference profiles. J. Neurosci. Methods 18 339–350 Occurrence Handle10.1016/0165-0270(86)90022-1 Occurrence Handle1:STN:280:BiiD1cfktVI%3D Occurrence Handle3540473
CA Shera JJ Guinan Jr (1998) ArticleTitleEvoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs. J. Acoust. Soc. Am. 105 782–798 Occurrence Handle10.1121/1.426948
MS Springer GC Cleven O Madsen WW de Jong VG Waddell HM Amrine MJ Stanhope (1997) ArticleTitleEndemic African mammals shake the phylogenetic tree. Nature 388 61–64 Occurrence Handle1:CAS:528:DyaK2sXksVSksbo%3D Occurrence Handle9214502
H Stephan G Baron HD Frahm (1991) Comparative Brain Research in Mammals: Insectivora. Springer Verlag New York
ML Whitehead BL Lonsbury–Martin GK Martin MJ McCoy (1996) Otoacoustic emissions: animal models and clinical observations. TR Van de Water AN Popper RR Fay (Eds) Clinical aspects of hearing. Springer-Verlag New York 199–257
FH Willard GF Martin (1983) ArticleTitleThe auditory brainstem nuclei and some of their projections to the inferior colliculus in the North American opossum. Neuroscience 10 1203–1232 Occurrence Handle10.1016/0306-4522(83)90109-4 Occurrence Handle1:STN:280:BiuC3MjotFA%3D Occurrence Handle6664491
Acknowledgements
H. Künzle placed tenrecs of his breeding colony at our disposal. The neurophysiological experiments were conducted in his laboratory. G. Schuller contributed during the startup to the design and realization of the neurophysiological experiments. The animal experiments comply with the “Principles of animal care,” publication No. 86-23, revised 1985, of the National Institutes of Health, and also with the German law (approved by the Regierung von Oberbayern, 211-2531-31/97 and 211-2531-37/98). This study was supported by the DFG Ku 624/2-1 and Ko 987/6-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drexl, M., Faulstich, M., von Stebut, B. et al. Distortion Product Otoacoustic Emissions and Auditory Evoked Potentials in the Hedgehog Tenrec, Echinops telfairi . JARO 4, 555–564 (2003). https://doi.org/10.1007/s10162-002-3043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-002-3043-5