Abstract
Background
Some clinical trials have shown that soluble urokinase-type plasminogen activator receptor (suPAR) has good predictive value for acute kidney injury (AKI), but there is still a lack of evidence-based proof. Therefore, we conducted this systematic review and meta-analysis to evaluate the predictive value of suPAR for AKI.
Methods
Pubmed, EMBASE, Cochrane Library, and Web of Science databases were searched until December 2021 to obtain the literature on the prediction of suPAR for AKI. The quality of the included studies was assessed using the QUADAS-2 scoring system, and a bivariate random-effect model was used for the meta-analysis. The present study has been registered on PROSPERO (Registration No. CRD42022324978).
Results
Seven articles were included, involving 2,319 patients, 635 of whom were AKI patients. The meta-analysis results showed that the combined sensitivity of suPAR in predicting AKI was 0.77 (95% CI 0.67–0.84); the specificity was 0.64 (95% CI 0.53–0.75); the odds ratio of diagnosis was 6 (95% CI 3–10); the pooled positive likelihood ratio was 2.2 (95% CI 1.6–2.9); the pooled negative likelihood ratio was 0.36 (95% CI 0.26–0.52); and the area under the summary receiver-operating characteristic (SROC) curve was 0.77 (95% CI 0.12~0.99). Deek’s funnel plot suggested no potential publication bias among included studies.
Conclusion
suPAR is a valuable biomarker for the prediction of AKI with relatively high predictive accuracy, but its clinical application needs improvements. SuPAR should be considered as an indicator in the subsequent development of more effective predictive tools for AKI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a common and severe clinical syndrome that occurs in approximately 0.15 of hospitalized patients, and its morbidity has been reported in more than half of patients in the intensive care unit (ICU), with a high mortality rate [1,2,3]. Currently, the diagnosis of AKI is still based on the rapid increase in serum (or plasma) creatinine, a decrease in urine volume, or both [4,5,6]. However, the concentration of creatinine is affected by multiple clinical variables, such as hydration, nutritional status, muscle metabolism, or drug effects, and serum creatinine does not alter until the glomerular filtration rate (GFR) is reduced by about 0.50 [7, 8]. Owing to these limitations, creatinine remains an insufficient predictor of AKI. As a result, there is an urgent need for an accurate and timely biomarker to predict the occurrence or progression of AKI.
Soluble urokinase plasminogen activator receptor (suPAR) is a novel biomarker. SuPAR molecules are derived from the proteolytic cleavage of membrane-bound urokinase plasminogen activator receptor (uPAR, cd87), and can be detected in different body fluids, such as blood, urine, peritoneal fluid, and cerebrospinal fluid. Moreover, suPAR is highly stable, and the concentration of suPAR in serum is not affected by diet, drugs, inflammation, and time of collection throughout the day [9]. SuPAR is a signal transduction glycoprotein that is believed to be involved in the pathogenesis of kidney diseases [10]. Previous studies have shown that the systemic inflammatory biomarker suPAR is an important biomarker for the early identification of AKI [11, 12]. Hayek et al. have recently found that elevated suPAR is associated with an increased risk of AKI in patients undergoing coronary angiography or cardiac surgery and in those hospitalized in intensive care units [10]. However, there is still no evidence that suPAR can be used as a marker to predict the occurrence of AKI, or that high suPAR levels are associated with AKI. Therefore, we conducted this systematic review and meta-analysis to evaluate the predictive value of suPAR for AKI.
Materials and methods
Literature retrieval
The search databases for this study were EMBASE, Cochrane Library, PubMed, and Web of Science, and the search time was as of December 2021. The search strategy was a combination of subject headings and free words, and minor adjustments might be made to each database. The search terms in PubMed included “Acute Kidney Injury” [Mesh], and “Receptors, Urokinase Plasminogen Activator [Mesh]”. The detailed retrieval strategy is shown in Appendix 1. The present study has been registered on PROSPERO (Registration No. CRD42022324978).
Literature inclusion and exclusion criteria
Inclusion criteria: (1) The literature type was cohort study, cross-sectional study, or diagnostic trial study; (2) English literature; (3) Studies containing the gold standard for the definite diagnosis of AKI and non-AKI cases; (4) Studies in which the four-grid diagnostic table could be directly or indirectly extracted from outcome indicators.
Exclusion criteria: (1) Non-clinical studies, such as reviews, medical records, and conference abstracts; (2) In vitro or animal experiments; (3) Studies from which the diagnostic four-grid table could not be directly or indirectly extracted.
Literature screening and data extraction
The EndNote software was used for literature management, and the literature was screened according to the inclusion and exclusion criteria. After the final included studies were determined, information extraction was performed, including the first author, country, sample size, AKI occurrence background, number of true positives, number of false positives, number of true negatives, number of false negatives, sensitivity, specificity, and ROC curve.
The literature screening and information extraction were carried out independently by two researchers (Y.H. and S.C.H.), and cross-examination was conducted after completion. If there was any disagreement, a third researcher (X.Y.Z.) was invited to assist in adjudication.
Quality evaluation
In the present study, two investigators (Y.H. and S.C.H.) independently used QUADAS-2 to assess the methodological quality of included studies. They cross-checked the results after the evaluation was completed. If there was any disagreement, a third investigator (X.Y.Z.) assisted in adjudication. The application of the QUADAS-2 has four phases: summarize the review question, tailor the tool and produce review-specific guidance, construct a flow diagram for the primary study, and judge bias and applicability.
Statistical analysis
A complete meta-analysis was performed using Stata 15.0 (Stata Corporation, College Station, TX), and a bivariate mixed model was used to pool effect sizes. The combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio with 95% confidence interval (95%CI) were analyzed. A sensitivity analysis was performed to evaluate the stability of meta-analysis results, and Deek’s funnel plot asymmetry test was adopted to assess publication bias. Owing to the small number of included studies, we did not perform meta-regression. In this study, p < 0.05 indicated that the difference was statistically significant.
Results
Retrieval results
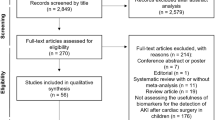
A total of 215 relevant studies were retrieved, 157 of which were left after removing duplicate or irrelevant literature. According to the title and abstract, 125 studies that did not meet the criteria were excluded. After a full-text review of the remaining 32 studies, 7 studies were finally included in this meta-analysis. The literature screening process is shown in Figure 1.
Basic information
The seven included studies were published between 2017 and 2021, with patients from four countries and multiple ethnicities, including two from Denmark [13, 14], three from Germany [12, 15, 16], one from China [17], and one from the United States [18]. The study design was mainly retrospective. A total of 2319 patients were included, comprising 635 AKI patients and 1684 non-AKI patients, and most of them were middle-aged and elderly. Most trials were done on critically ill patients, including those who had undergone cardiac surgery (extracorporeal circulation), elderly patients in the emergency department, patients in the ICU after colostomy or with sepsis, and hospitalized adults with COVID-19. The basic characteristics of all the included literature are presented in Table 1.
Methodological quality evaluation
According to the evaluation items of QUADAS-2, the risk of bias was assessed in four domains: case selection, trial to be evaluated, reference standard, and flow and timing. The clinical applicability of the first three domains was evaluated at the same time. The risk of bias assessment showed that the quality of all included studies was relatively good, and the high risk items were mainly in the case selection domain, as shown in Figure 2.
Meta-analysis results
The pooled sensitivity of suPAR in predicting AKI was 0.77 (95%CI 0.67–0.84); the pooled specificity was 0.64 (95%CI 0.53–0.75); the pooled predictive odds ratio was 6 (95%CI 3–10); the pooled positive likelihood ratio was 2.2 (95% CI 1.6–2.9); the pooled negative likelihood ratio was 0.36 (95% CI 0.26–0.52), and the area under the summary receiver-operating characteristic (SROC) curve was 0.77 (95% CI 0.12–0.99). The sensitivity, specificity, and SROC are presented in Figure 3.
In this meta-analysis, the heterogeneity I2 was 0.93 (95% CI 88–99), and the box plot revealed that the studies of Anne Byriel Walls [13] and Yuhan Qin [17] might be the main sources of heterogeneity. In addition, Deek’s funnel plot showed no apparent publication bias among the studies (p = 0.06). The bivariate boxplot for heterogeneity is shown in Figure 4A, and Deek’s funnel plot is shown in Figure 4B.
Fagan’s plot was employed to reflect the clinical applicability of suPAR in predicting AKI. The incidence of AKI varies significantly in different contexts and even at different income levels. It is estimated to range from 0.01 to 0.66, and exceeds 0.50 in the ICU [1, 19]. Therefore, assuming a prior probability of 0.27, the probability of the diagnosis of AKI is 0.46 in the case of a positive likelihood ratio of 2, and the probability of the diagnosis of no AKI is 0.12 in the case of a negative likelihood ratio of 0.35. The clinical application is shown in Figure 5.
Discussion
This meta-analysis showed that suPAR could be used as a predictor of AKI, with a comprehensive sensitivity of 0.77 and a comprehensive specificity of 0.64. There was no significant publication bias among the included studies. The prediction effect of suPAR seemed to be different in various AKI occurrence backgrounds. Among the seven included articles, the sensitivity of serum suPAR in the diagnosis of AKI ranged from 0.63 to 0.90, and the specificity varied from 0.40 to 0.83, with significant difference. This may be attributed to the differences in the research design and experimental methods in response to various population levels or diseases, resulting in different detection thresholds. The testing instruments and diagnostic reagents used in different countries were not identical, and differences also existed at the operator level. The box plot for heterogeneity reflected that the studies of Anne Byriel Walls [13] and Yuhan Qin [17] might be the main sources of heterogeneity.
In the recent years, the discovery and application of new biomarkers for early diagnosis of AKI have become one of the hot spots in kidney disease research. A previous study showed that plasma suPAR was an incidental phenomenon associated with the inflammatory shedding of receptors on neutrophils, monocytes, and macrophages [20]. A mechanistic study on models of kidney disease showed that circulating suPAR (derived from inflammatory cells) interacted with αvβ3 integrins on podocytes, and elevated plasma suPAR levels could predict the occurrence of nephropathy in seemingly healthy individuals and those at risk of chronic kidney disease [21]. This suggested that suPAR played a role in the pathogenesis of AKI. Targeting suPAR has therapeutic promise as it is pathogenic. In experimental mouse models, the use of anti-suPAR MABs eliminated the adverse effects of suPAR on the kidney, indicating that suPAR is a promising therapeutic target for alleviating AKI [10, 22, 23]. Therefore, eliminating suPAR from circulation or neutralizing its biological effects may be a reasonable strategy to reduce the incidence, morbidity, and mortality in AKI. In clinical trials, biomarkers such as suPAR have the advantage of identifying AKI at an early stage and predicting AKI [24].
In this study, Fagan’s plot was used to reflect the clinical applicability of suPAR in predicting AKI. Assuming that the probability before diagnosis is 0.50, the probability of the diagnosis of AKI is 0.68 in the case of a positive likelihood ratio of 2, and the probability of the diagnosis of no AKI is 0.27 in the case of a negative likelihood ratio of 0.36. Most importantly, if AKI can be predicted, there will be more testable therapeutics, since the majority of AKI interventions identified in preclinical studies are effective only when implemented before injury[25]. The current biomarkers under study, such as neutrophil gelatinase-associated lipid calin (NGAL), kidney injury molecule 1, urinary IL 18, and plasma cystatin C, are also early markers of AKI [26]. Studies have found that ROC analysis of suPAR and NGAL yielded an area under curve (AUC) of 0.69 and 0.78, respectively, without a significant difference (p = 0.117). The AUC of suPAR combined with NGAL was 0.80, significantly higher than that of suPAR alone (p = 0.032) [13]. Furthermore, tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) were found to be promising biomarkers for risk stratification in infectious AKI patients requiring renal replacement therapy (RRT) and predictors of AKI in out-of-hospital cardiac arrest survivors [27]. Compared with [TIMP-2] × [IGFBP7], baseline suPAR values have already been reported to have the ability to predict the demand for RRT with good diagnostic accuracy. Urinary [TIMP-2] × [IGFBP7] can be used as an early predictor of moderate and severe AKI as well as a potential tool to monitor the treatment of Kidney-oriented sepsis [28, 29]. Therefore, combining multiple markers can more effectively predict AKI and provide more accurate predictive value for clinical practice. Several studies have demonstrated that using these biomarkers in combination with recommended AKI care practices (e.g., avoidance of nephrotoxins and optimization of hemodynamics) can improve patients’ prognosis [28, 30].
This study has the following advantages. First, it is the first to discuss the predictive value of suPAR for AKI. The analysis results show that suPAR or its related indicators should be considered in the subsequent development or optimization of AKI prediction tools/scoring systems. Second, the quality of the included literature is ideal, and there is no bias in the results based on the data. Meanwhile, this study also has the following limitations. On the one hand, although we conducted a comprehensive and systematic search, there are still few studies included in this meta-analysis. On the other hand, with a limited number of included studies, there is no sufficient evidence to support our discussion on the predictive effect of AKI under different occurrence backgrounds.
Conclusion
SuPAR may be a valuable biomarker for predicting AKI, suggesting that suPAR or its related indicators should be considered in subsequent development or optimization of predictive tools/scoring systems for AKI. Although a comprehensive search was performed, the number of included articles is relatively small. Large-scale studies are desired to verify our findings in the future.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
References
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet (London, England). 2019;394(10212):1949–64. https://doi.org/10.1016/s0140-6736(19)32563-2.
Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14(4):217–30. https://doi.org/10.1038/nrneph.2017.184.
Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Internal Med. 2010;268(3):296–308. https://doi.org/10.1111/j.1365-2796.2010.02252.x.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care (London, England). 2004;8(4):R204-12. https://doi.org/10.1186/cc2872.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care (London, England). 2007;11(2):R31. https://doi.org/10.1186/cc5713.
Disease KJKIS. Improving global outcomes (KDIGO) acute kidney injury work group: KDIGO clinical practice guideline for acute kidney injury. kidney Int Suppl. 2012;2:1–138.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. New Engl J Med. 2006;354(23):2473–83. https://doi.org/10.1056/NEJMra054415.
de Geus HR, Betjes MG, Bakker J. Biomarkers for the prediction of acute kidney injury: a narrative review on current status and future challenges. Clin Kid J. 2012;5(2):102–8. https://doi.org/10.1093/ckj/sfs008.
Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80(2):209–16. https://doi.org/10.1002/jmv.21114.
Hayek SS, Leaf DE, Samman Tahhan A, Raad M, Sharma S, Waikar SS, et al. Soluble urokinase receptor and acute kidney injury. New Engl J Med. 2020;382(5):416–26. https://doi.org/10.1056/NEJMoa1911481.
Iversen E, Houlind MB, Kallemose T, Rasmussen LJH, Hornum M, Feldt-Rasmussen B, et al. Elevated suPAR Is an independent risk marker for incident kidney disease in acute medical patients. Front Cell Dev Biol. 2020;8:339. https://doi.org/10.3389/fcell.2020.00339.
Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017;18(8):1662. https://doi.org/10.3390/ijms18081662.
Walls AB, Bengaard AK, Iversen E, Nguyen CN, Kallemose T, Juul-Larsen HG, et al. Utility of suPAR and NGAL for AKI risk stratification and early optimization of renal risk medications among older patients in the emergency department. Pharmaceuticals (Basel, Switzerland). 2021;14(9):843. https://doi.org/10.3390/ph14090843.
Rasmussen SR, Nielsen RV, Møgelvang R, Ostrowski SR, Ravn HB. Prognostic value of suPAR and hsCRP on acute kidney injury after cardiac surgery. BMC Nephrol. 2021;22(1):120. https://doi.org/10.1186/s12882-021-02322-0.
Loosen SH, Tacke F, Binnebosel M, Leyh C, Vucur M, Heitkamp F, et al. Serum levels of soluble urokinase plasminogen activator receptor (suPAR) predict outcome after resection of colorectal liver metastases. Oncotarget. 2018;9(43):27027–38. https://doi.org/10.18632/oncotarget.25471.
Nusshag C, Rupp C, Schmitt F, Krautkrämer E, Speer C, Kälble F, et al. Cell cycle biomarkers and soluble urokinase-type plasminogen activator receptor for the prediction of sepsis-induced acute kidney injury requiring renal replacement therapy: A prospective, exploratory study. Crit Care Med. 2019;47(12):e999–1007. https://doi.org/10.1097/ccm.0000000000004042.
Qin Y, Qiao Y, Wang D, Yan G, Tang C, Ma G. The predictive value of soluble urokinase-type plasminogen activator receptor in contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Int J Gen Med. 2021;14:6497–504. https://doi.org/10.2147/ijgm.S339075.
Azam TU, Shadid HR, Blakely P, O’Hayer P, Berlin H, Pan M, et al. Soluble Urokinase Receptor (SuPAR) in COVID-19-Related AKI. J Am Soc Nephrol JASN. 2020;31(11):2725–35. https://doi.org/10.1681/asn.2020060829.
Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–25. https://doi.org/10.1038/s41581-018-0052-0.
Gussen H, Hohlstein P, Bartneck M, Warzecha KT, Buendgens L, Luedde T, et al. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:26. https://doi.org/10.1186/s40560-019-0381-5.
Hayek SS, Sever S, Ko Y-A, Trachtman H, Awad M, Wadhwani S, et al. Soluble Urokinase Recept Chronic Kidney Dis. 2015;373(20):1916–25.
Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. https://doi.org/10.1038/nm1696.
Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23(1):100–6. https://doi.org/10.1038/nm.4242.
Faubel S. SuPAR: a potential predictive biomarker for acute kidney injury. Nat Rev Nephrol. 2020;16(7):375–6. https://doi.org/10.1038/s41581-020-0276-7.
Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD. Ongoing clinical trials in AKI. Clin J Am Soc Nephrol CJASN. 2012;7(5):861–73. https://doi.org/10.2215/cjn.12191111.
Griffin BR, Gist KM, Faubel S. Current status of novel biomarkers for the diagnosis of acute kidney injury: a historical perspective. J Intensive Care Med. 2020;35(5):415–24. https://doi.org/10.1177/0885066618824531.
Adler C, Heller T, Schregel F, Hagmann H, Hellmich M, Adler J, et al. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Crit Care (London, England). 2018;22(1):126. https://doi.org/10.1186/s13054-018-2042-9.
Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43(11):1551–61. https://doi.org/10.1007/s00134-016-4670-3.
Fiorentino M, Xu Z, Smith A, Singbartl K, Palevsky PM, Chawla LS, et al. Serial measurement of cell-cycle arrest biomarkers [TIMP-2] · [IGFBP7] and risk for progression to death, dialysis, or severe acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2020;202(9):1262–70. https://doi.org/10.1164/rccm.201906-1197OC.
Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, et al. A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int. 2020;97(3):580–8. https://doi.org/10.1016/j.kint.2019.10.015.
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Funding
This study was funded by Peak Climbing Project of Foshan Hospital of Traditional Chinese Medicine. ID: (202000205)
Author information
Authors and Affiliations
Contributions
YH and SH: wrote the main manuscript and fully participated in all analyses. XZ and SH: contributed to the study concept and design. YH and ML: participated in literature search, data extraction, and quality assessment. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Huang, Y., Huang, S., Zhuo, X. et al. Predictive value of suPAR in AKI: a systematic review and meta-analysis. Clin Exp Nephrol 27, 1–11 (2023). https://doi.org/10.1007/s10157-022-02300-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-022-02300-2