Abstract
Background
There is no evidence regarding appropriate target hemoglobin levels in chronic kidney disease (CKD) patients with an erythropoiesis-stimulating agent (ESA)-hyporesponsiveness. Therefore, we conducted a randomized controlled study in non-dialysis dependent CKD (NDD-CKD) patients with ESA-hyporesponsiveness, comparing results of intensive versus conservative treatment to maintain hemoglobin levels.
Methods
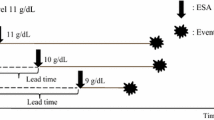
This was a multicenter, open-label, randomized, parallel-group study conducted at 89 institutions. Among NDD-CKD patients, those with ESA-hyporesponsive renal anemia were randomly assigned to an intensive treatment group, to which epoetin beta pegol was administered with target hemoglobin level of 11 g/dL or higher, or conservative treatment group, in which the hemoglobin levels at enrollment (within ± 1 g/dL) were maintained. The primary endpoint was the time to the first kidney composite event defined as (1) transition to renal replacement therapy (dialysis or renal transplantation); (2) reduction of estimated glomerular filtration rate (eGFR) to less than 6.0 mL/min/1.73 m2; or (3) reduction of eGFR by 30% or more. Secondary endpoints were kidney function (change rate in eGFR), cardiovascular (CV) events, and safety.
Results
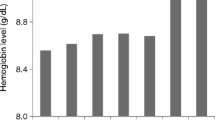
Between August 2012 and December 2015, 385 patients were registered, and 362 patients who met the eligibility criteria were enrolled. There was no significant difference in kidney survival or in CV events between the two groups. However, the incidences of the 3 types of kidney composite events tended to differ.
Conclusions
In NDD-CKD patients with ESA-hyporesponsive renal anemia, the aggressive administration of ESA did not clearly extend kidney survival or result in a significant difference in the incidence of CV events.




Similar content being viewed by others
References
Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ren Rep Ther. 2017;3:36.
Japanese Society of Nephrology. Essential points from Evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23:1–15.
Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98.
Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–32.
Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–84.
Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, et al. Secondary analysis of the CHOIR trial epoetin-alfa dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–8.
Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363:1146–55.
Tsubakihara Y, Itami N, Uda S, Shimizu H, Naruse T, Hayashi T, et al. Maintenance of improvement in anemia with subcutaneously injected continuous erythropoiesis receptor activator in predialysis patients with chronic kidney disease, using recombinant human erythropoietin as the control. Kidney Dial. 2011;70:953–63 (Japanese article).
Kuwahara M, Mandai S, Kasagi Y, Kusaka K, Tanaka T, Shikuma S, et al. Responsiveness to erythropoiesis-stimulating agents and renal survival in patients with chronic kidney disease. Clin Exp Nephrol. 2015;19:598–605.
Kainz A, Mayer B, Kramar R, Oberbauer R. Association of ESA hypo-responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrol Dial Transplant. 2010;25:3701–6.
Fukuma S, Yamaguchi T, Hashimoto S, Nakai S, Iseki K, Tsubakihara Y, et al. Erythropoiesis-stimulating agent responsiveness and mortality in hemodialysis patients: results from a cohort study from the dialysis registry in Japan. Am J Kidney Dis. 2012;59:108–16.
Ishigami J, Onishi T, Shikuma S, Akita W, Mori Y, Asai T, et al. The impact of hyporesponsiveness to erythropoietin-stimulating agents on time-dependent mortality risk among CKD stage 5D patients: a single-center cohort study. Clin Exp Nephrol. 2013;17:106–14.
Okazaki M, Komatsu M, Kawaguchi H, Tsuchiya K, Nitta K. Erythropoietin resistance index and the all-cause mortality of chronic hemodialysis patients. Blood Purif. 2014;37:106–12.
Eriguchi R, Taniguchi M, Ninomiya T, Hirakata H, Fujimi S, Tsuruya K, et al. Hyporesponsiveness to erythropoiesis-stimulating agent as a prognostic factor in Japanese hemodialysis patients: the Q-Cohort study. J Nephrol. 2015;28:217–25.
Luo J, Jensen DE, Maroni BJ, Brunelli SM. Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis. 2016;68:763–71.
Bae MN, Kim SH, Kim YO, Jin DC, Song HC, Choi EJ, et al. Association of erythropoietin-stimulating agent responsiveness with mortality in hemodialysis and peritoneal dialysis patients. PLoS ONE. 2015;10:e0143348.
Guerrero-Riscos MÁ, Montes-Delgado R, Seda-Guzmán M, Praena-Fernández JM. Erythropoietin resistance and survival in non-dialysis patients with stage 4–5 chronic kidney disease and heart disease. Nefrologia. 2012;32:343–52.
Minutolo R, Conte G, Cianciaruso B, Bellizzi V, Camocardi A, De Paola L, et al. Hyporesponsiveness to erythropoiesis-stimulating agents and renal survival in non-dialysis CKD patients. Nephrol Dial Transplant. 2012;27:2880–6.
Tsuruya K, Uemura Y, Hirakata H, Kitazono T, Tsubakihara Y, Suzuki M, et al. Association between responsiveness to methoxy polyethylene glycol-epoetin beta and renal survival in patients with non-dialysis-dependent chronic kidney disease: a pooled analysis of individual patient-level data from clinical trials. Nephrology (Carlton). 2017;22:769–75.
Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial. 2012;16:529–40.
Tanaka T, Nangaku M, Imai E, Tsubakihara Y, Kamai M, Wada M, et al. Safety and effectiveness of long-term use of darbepoetin alfa in non-dialysis patients with chronic kidney disease: a post-marketing surveillance study in Japan. Clin Exp Nephrol. 2019;23:231–43.
Hayashi T, Uemura Y, Kumagai M, Kimpara M, Kanno H, Ohashi Y, MIRACLE-CKD Study Group. Effect of achieved hemoglobin level on renal outcome in non-dialysis chronic kidney disease (CKD) patients receiving epoetin beta pegol: MIRcerA CLinical Evidence on Renal Survival in CKD patients with renal anemia (MIRACLE-CKD Study). Clin Exp Nephrol. 2019;23:349–61.
Tanaka K, Watanabe T, Takeuchi A, Ohashi Y, Nitta K, Akizawa T, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91:227–34.
Denker M, Boyle S, Anderson AH, Appel LJ, Chen J, Fink JC, et al. Chronic renal insufficiency cohort study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10:2073–83.
Hiram-Bab S, Liron T, Deshet-Unger N, Mittelman M, Gassmann M, Rauner M, et al. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. 2015;29:1890–900.
Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, et al. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE. 2010;5:e10853.
Acknowledgements
The authors would like to thank Drs. Hiroshi Ito, Takanari Kitazono and Takashi Wada for evaluation of kidney and CV events; Drs. Masashi Suzuki, Yoshiharu Tsubakihara and Satoshi Morita for independent data monitoring; and the many investigators, nurses and clinical research coordinators who contributed to this study.
Funding
The funding for this study was provided by Chugai Pharmaceutical Co., Ltd., on the basis of a commissioning contract, and was provided to EP-CRSU Co., Ltd. (Japanese Contract Research Organization) that handled administration tasks, etc., for the study secretariat and data center. The funding party, Chugai Pharmaceutical Co., was involved with the conception of the study, and provision of information, but not with planning or conducting the study, or analyzing or interpreting the results.
Author information
Authors and Affiliations
Consortia
Contributions
HH leaded this study as principal investigator. HH, HY, KT, HH, SN, MN, TW, T, YU and YO participated in the interpretation of study results, and were involved in this study design, and approval of the manuscript. HH, HY, KT, HH, SN, MN, TW and TH were investigators of this study. YO and YU conducted the statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
K. Tsuruya has received speakers fee as honoraria and grants from Chugai and Kyowa Kirin. T. Hayashi has received speakers fee as honoraria from Chugai and Kyowa Kirin. H. Yamamoto has received speakers fee as honoraria from Chugai and Kyowa Kirin. H. Hase has received grants from Chugai and Kyowa Kirin. S. Nishi has received speakers fee as honoraria and grants from Chugai and Kyowa Kirin. K. Yamagata has received speakers fee as honoraria and grants from Chugai and Kyowa Kirin. M. Nangaku has received speakers fee as honoraria from Chugai, Kyowa Kirin and Kissei, grants from Chugai and Kyowa Kirin, research funding from Kyowa Kirin and manuscript fee from Kyowa Kirin. T. Wada has received grants from Chugai and Kyowa Kirin, and research funding from Kyowa Kirin. Y. Uemura has received speakers fee as honoraria from Chugai. Y. Ohashi has received speakers fee as honoraria from Chugai. H. Hirakata has speakers fee as honoraria from Chugai.
Human rights
This study was conducted in compliance with the Declaration of Helsinki and “Ethical Guidelines for Clinical Studies” by the Ministry of Health, Labour and Welfare and in accordance with International Council for Harmonization Good Clinical Practice (ICH-GCP) guidelines. The study was approved by an independent central ethics committee and was registered in the University Hospital Medical Information Network (UMIN) database (UMIN000008617). Study treatments performed were covered by ordinary health insurance. This report was prepared according to the Consolidated Standards of Reporting Trials (CONSORT).
Informed consent
Written informed consent was obtained from all included participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tsuruya, K., Hayashi, T., Yamamoto, H. et al. Renal prognoses by different target hemoglobin levels achieved by epoetin beta pegol dosing to chronic kidney disease patients with hyporesponsive anemia to erythropoiesis-stimulating agent: a multicenter open-label randomized controlled study. Clin Exp Nephrol 25, 456–466 (2021). https://doi.org/10.1007/s10157-020-02005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-020-02005-4