Abstract
Background
Prothymosin alpha (ProTα) is a nuclear protein expressed in virtually all mammalian tissues. Previous studies have shown that ProTα exhibits protective effects against ischemia-induced cell death in various cell types. Recently, the 6-residue peptide P6Q (NEVDQE), the modified form of the active 6-residue core (51–56) in ProTα, has also been shown to have protective effects against retinal ischemia. However, it remains to be elucidated whether P6Q is effective against acute kidney injury (AKI). Therefore, we investigated the renoprotective effect of P6Q on cisplatin-induced AKI.
Methods
Cultured HK-2 cells were treated with cisplatin for 24 h and pretreatment with ProTα or P6Q was carried out 30 min before cisplatin treatment. Cell viability was evaluated using the MTT assay. In an in vivo study, 8-week-old male Wistar rats were divided into control, cisplatin treated, and cisplatin treated with P6Q injection groups. In the last of these, P6Q was injected intravenously before cisplatin treatment. Then, we evaluated the renoprotective effect of P6Q.
Results
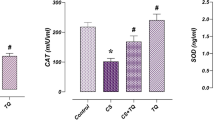
In the study on cultured cells, pretreatment with ProTα or P6Q prevented cisplatin-induced cell death. In the in vivo study, pretreatment with P6Q significantly attenuated cisplatin-induced increase in serum creatinine and blood urea nitrogen levels, renal tubular cell injury, and apoptosis. Moreover, P6Q attenuated the mitochondrial apoptotic pathway and accelerated Akt phosphorylation after cisplatin-induced renal damage.
Conclusion
Taken together, our findings indicate that P6Q can attenuate cisplatin-induced AKI and suppress the mitochondrial apoptotic pathway via Akt phosphorylation. These data suggest that P6Q has potential as a preventative drug for cisplatin-induced AKI.





Similar content being viewed by others
References
Dasari S, Bernard TP. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20.
Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–67.
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2:2490–518.
Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–24.
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;7:994–1007.
Cannavo A, Rengo G, Liccardo D, Pironti G, Scimia MC, Scudiero L, De Lucia C, Ferrone M, Leosco D, Zambrano N, Koch WJ, Trimarco B, Esposito G. Prothymosin alpha protects cardiomyocytes against ischemia-induced apoptosis via preservation of Akt activation. Apoptosis. 2013;18:1252–61.
Mosoian A. Intracellular and extracellular cytokine-like functions of prothymosin α: implications for the development of immunotherapies. Future Med Chem. 2011;3:1199–208.
Emmanouilidou A, Karetsou Z, Tzima E, Kobayashi T, Papamarcaki T. Knockdown of prothymosin α leads to apoptosis and developmental defects in zebrafish embryos. Biochem Cell Biol. 2013;91:325–32.
Ioannou K, Samara P, Livaniou E, Derhovanessian E, Tsitsilonis OE. Prothymosin alpha: a ubiquitous polypeptide with potential use in cancer diagnosis and therapy. Cancer Immunol Immunother. 2012;61:599–614.
Jiang X, Kim H-E, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X. Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science. 2003;299:223–6.
Ueda H, Matsunaga H, Halder SK. Prothymosin α plays multifunctional cell robustness roles in genomic, epigenetic, and nongenomic mechanisms. Ann N Y Acad Sci. 2012;1269:34–43.
Halder SK, Matsunaga H, Ishii KJ, Ueda H. Prothymosin-alpha preconditioning activates TLR4-TRIF signaling to induce protection of ischemic retina. J Neurochem. 2015;135:1161–77.
Halder SK, Matsunaga H, Yamaguchi H, Ueda H. Novel neuroprotective action of prothymosin alpha-derived peptide against retinal and brain ischemic damages. J Neurochem. 2013;125:713–23.
Ueda H, Halder SK, Matsunaga H, Sasaki K, Maeda S. Neuroprotective impact of prothymosin alpha-derived hexapeptide against retinal ischemia-reperfusion. Neuroscience. 2016;318:206–18.
Minhas G, Sharma J, Khan N. Cellular stress response and immune signaling in retinal ischemia-reperfusion injury. Front Immunol. 2016;7:444.
Katai N, Yoshimura N. Apoptotic retinal neuronal death by ischemia-reperfusion is executed by two distinct caspase family proteases. Investig Ophthalmol Vis Sci. 1999;40:2697–705.
Song N, Endo D, Song B, Shibata Y, Koji T. 5-aza-2′-deoxycytidine impairs mouse spermatogenesis at multiple stages through different usage of DNA methyltransferases. Toxicology. 2016;361–362:62–72.
Miyaji T, Kato A, Yasuda H, Fujigaki Y, Hishida A. Role of the increase in p21 in cisplatin-induced acute renal failure in rats. J Am Soc Nephrol. 2001;12:900–8.
Qi X, Wang L, Du F. Novel small molecules relieve prothymosin alpha-mediated inhibition of apoptosome formation by blocking its interaction with Apaf-1. Biochemistry. 2010;49:1923–30.
Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell. 2006;10:549–61.
Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–6.
Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483–94.
dos Santos NAG, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–50.
Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82(5):516–24.
Suzuki S, Takahashi S, Takahashi S, Takeshita K, Hikosaka A, Wakita T, Nishiyama N, Fujita T, Okamura T, Shirai T. Expression of prothymosin alpha is correlated with development and progression in human prostate cancers. Prostate. 2006;66:463–9.
Lin YT, Liu YC, Chao CCK. Inhibition of JNK and prothymosin-alpha sensitizes hepatocellular carcinoma cells to cisplatin. Biochem Pharmacol. 2016;122:80–9.
Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86.
Acknowledgements
We thank Ms. Ryoko Yamamoto for excellent experimental assistance.
Funding
This research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Human and animal rights
This article does not contain any studies with human participants.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (IRB approval number 1506261242-5).
Informed consent
This section is not applicable to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Torigoe, K., Obata, Y., Torigoe, M. et al. Hexapeptide derived from prothymosin alpha attenuates cisplatin-induced acute kidney injury. Clin Exp Nephrol 24, 411–419 (2020). https://doi.org/10.1007/s10157-019-01843-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-019-01843-1