Abstract
Background
Cirrhosis of the liver is often associated with an impairment of renal function that is usually not associated with consistent structural abnormalities of the renal parenchyma, but is thought to be the functional consequence of arterial underfilling and reduced arterial blood pressure.
Method
We have used the cirrhosis model of chronic bile duct ligation (BDL) to assess the response of renal blood flow to a change of blood pressure. We have measured renal haemodynamics in BDL rats.
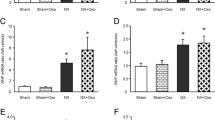
Result
Three weeks after BDL, rats showed elevated levels of total bilirubin, AST, and ALT as well as reduced arterial blood pressure. Creatinine clearance was significantly reduced, and plasma creatinine and urea nitrogen were elevated. Renal blood flow at baseline blood pressure was significantly lower in the BDL group than in the sham group. Clamp-induced reductions of renal perfusion pressure caused significantly greater changes of renal blood flow in BDL than control rats. The autoregulatory index over a comparable blood pressure range averaged 0.28 ± 0.35 in control rats and 1.26 ± 0.6 in BDL rats (p = 0.0004) indicating impairment of renal autoregulation in liver cirrhosis.
Conclusion
Tubuloglomerular feedback (TGF) responses were significantly attenuated in BDL rats, especially in the subnormal flow range. Impairment of renal blood flow autoregulation, to some extent mediated by reduced TGF-mediated vasodilatation, may contribute to the renal vascular constrictor state in liver cirrhosis by preventing the full dilatory response to the blood pressure reduction.





Similar content being viewed by others
References
Hirata M, Harihara Y, Sano K, Kusaka K, Kita Y, Nakao A, Taniguchi S, Seki G, Yoshino H, Ito M, Kubota K, Takayama T, Kawarasaki H, Hashizume K, Makuuchi M. Recovery of renal function after living-related liver transplantation in a case of hepatitis B virus liver cirrhosis with renal failure. Transplant Proc. 1999;31:2904–5.
Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med. 1969; 280:1367–71.
Kramer L, Horl WH. Hepatorenal syndrome. Semin Nephrol. 2002;22:290–301.
Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol. 1987;49:251–73.
Hashimoto S, Kawata T, Schnermann J, Koike T. Chloride channel blockade attenuates the effect of angiotensin II on tubuloglomerular feedback in WKY but not spontaneously hypertensive rats. Kidney Blood Press Res. 2004;27:35–42.
Hashimoto S, Yamada K, Kawata T, Mochizuki T, Schnermann J, Koike T. Abnormal autoregulation and tubuloglomerular feedback in prediabetic and diabetic OLETF rats. Am J Physiol Renal Physiol. 2009;296:F598-604.
Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10: 2569–76.
Pereira RM, dos Santos RA, Oliveira EA, Leite VH, Dias FL, Rezende AS, Costa LP, Barcelos LS, Teixeira MM, Simoes e Silva AC. Development of hepatorenal syndrome in bile duct ligated rats. World J Gastroenterol. 2008;14:4505–11.
Rivera-Huizar S, Rincon-Sanchez AR, Covarrubias-Pinedo A, Islas-Carbajal MC, Gabriel-Ortiz G, Pedraza-Chaverri J, Alvarez-Rodriguez A, Meza-Garcia E, Armendariz-Borunda J. Renal dysfunction as a consequence of acute liver damage by bile duct ligation in cirrhotic rats. Exp Toxicol Pathol. 2006;58:185–95.
Wunderlich P, Hermle M, Davis JM, Mihatsch M, Brunner F, Thiel G. The role of tubuloglomerular feedback in acute impairment of renal function in obstructive jaundice. Clin Exp Dial Apheresis. 1983;7:63–76.
Wasser S, Tan CE. Experimental models of hepatic fibrosis in the rat. Ann Acad Med Singap. 1999;28:109–11.
Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015; 10;(96). https://doi.org/10.3791/52438.
Assimakopoulos SF, Vagianos CE. Bile duct ligation in rats: a reliable model of hepatorenal syndrome? World J Gastroenterol. 2009;15: 121–3.
Kotzampassi K, Metaxas G, Paramythiotis D, Pidonia I, Rekka H, Karamouzis M, Eleftheriadis E. The influence of continuous seven-day elevated intra-abdominal pressure in the renal perfusion in cirrhotic rats. J Surg Res. 2003; 115: 133–8.
Tunon MJ, Alvarez M, Culebras JM, Gonzalez-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086–98.
Wu J, Yang T, Wang C, Liu Q, Yao J, Sun H, Kaku T, Liu KX. Laennec protects murine from concanavalin A-induced liver injury through inhibition of inflammatory reactions and hepatocyte apoptosis. Biol Pharm Bull. 2008;31:2040–4.
Baker OS, Massry SG. Effect of chronic bile duct obstruction on renal handling of salt and water. J Clin Invest. 1972; 51: 402–11.
Bennett WM, Keeffe E, Melnyk C, Mahler D, Rosch J, Porter GA. Response to dopamine hydrochloride in the hepatorenal syndrome. Arch Intern Med. 1975;135:964–71.
Bradley SE. Editorial. Hepatorenal and glomerulotubular imbalance. N Engl J Med. 1973; 289: 1194–5.
Yang KT, Lin KE, Wu SS, Wang DJ. Uptake of Tc-99m MDP by renal cortex in a patient with advanced hepatic disease and oliguria. Clin Nucl Med. 1992;17:143.
Lichtmann IE. The substratum of memory. Dis Nerv Syst. 1960;21:350–1.
Bernardi M, Trevisani F, Gasbarrini A, Gasbarrini G. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis. 1994;14:23–34.
Veelken R, Hilgers KF, Porst M, Krause H, Hartner A, Schmieder RE. Effects of sympathetic nerves and angiotensin II on renal sodium and water handling in rats with common bile duct ligature. Am J Physiol Renal Physiol. 2005;288:F1267-75.
Epstein M, Goligorsky MS. Endothelin and nitric oxide in hepatorenal syndrome: a balance reset. J Nephrol. 1997;10:120–35.
Kayali Z, Herring J, Baron P, Franco E, Ojogho O, Smith J, Watkins G, Smith D, Lamin V, Hoang T, Sharma R, Mathahs M, Sowers L, Brown KE, Schmidt WN. Increased plasma nitric oxide, L-arginine, and arginase-1 in cirrhotic patients with progressive renal dysfunction. J Gastroenterol Hepatol. 2009; 24:1030–7.
Sansoe G, Silvano S, Mengozzi G, Smedile A, Touscoz G, Rosina F, Rizzetto M. Loss of tubuloglomerular feedback in decompensated liver cirrhosis: physiopathological implications. Dig Dis Sci. 2005;50:955–63.
Takabatake T, Ohta H, Hara H, Ishida Y, Noda Y, Hattori N. Activation of tubuloglomerular feedback in rat nephrons by sera from rabbits with fulminant hepatic failure. Nephron. 1983;35:136–9.
Wunderlich PF, Brunner FP, Davis JM, Haberle DA, Tholen H, Thiel G. Feedback activation in rat nephrons by sera from patients with acute renal failure. Kidney Int. 1980;17:497–506.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All animal experiments were conducted in accordance with “Hokkaido University Animal Experiment Guidelines”. And all animal experiments were receiving research approval from Hokkaido University animal experiment facility. (2008 − 168).
Informed consent
No relationship. All experiments were done on animals.
About this article
Cite this article
Maoka, T., Kawata, T., Koike, T. et al. Defective renal autoregulation in the chronic bile duct ligation model of liver failure. Clin Exp Nephrol 22, 1052–1060 (2018). https://doi.org/10.1007/s10157-018-1551-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-018-1551-9