Abstract
Background
TJN-331 is an inhibitor of transforming growth factor β1 (TGF-β1) production that has similar structural features to the natural product acteoside. This study was performed to examine the antinephritic effects of TJN-331 in a mouse model of experimental IgA nephropathy.
Materials and methods
IgA nephropathy was induced in ddY mice by oral administration of bovine γ globulin, followed by reticuloendothelial blocking by colloidal carbon injection and heminephrectomy. Effects of TJN-331 were examined over oral administration periods from 10 to 15 weeks after the third colloidal carbon injection. Intravenous administration of a TGF-β1-neutralizing antibody was used to investigate the role of TGF-β1 in IgA nephropathy.
Results
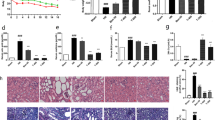
Administration of TJN-331 or captopril prevented elevation of serum creatinine. Histopathological examination after both experimental periods showed that TJN-331 inhibited increases in the mesangial matrix index and the number of nuclei per glomerular cross-section, compared with in untreated ddY mice with IgA nephropathy. TJN-331 prevented increase in glomerular TGF-β1 staining without affecting IgA. In the in vitro study, TJN-331 prevented total TGF-β1 production from splenocytes stimulated with concanavalin A. A neutralizing antibody against TGF-β1 prevented increase in the mesangial matrix index and the number of glomerular cells per cross-sectional area.
Conclusion
These results suggest that TJN-331 is effective against IgA nephropathy in ddY mice and acts via suppression of TGF-β1 production in glomeruli.






Similar content being viewed by others
References
Nogaki F, Oida E, Kamata T, Kobayashi I, Nomura K, Suyama K, et al. Chromosomal mapping of hyperserum IgA and glomerular IgA deposition in a high IgA (HIGA) strain of ddY mice. Kidney Int. 2005;68:2517–25.
Tomino Y. IgA nephropathy: lessons from an animal model, the ddY mouse. J Nephrol. 2008;21:463–7.
Sato M, Ideura T, Koshikawa S. Experimental IgA nephropathy in mice. Lab Invest. 1986;54:377–84.
Kusano H, Muso E, Ono T, Nogaki F, Nomura K, Takeda T, et al. Heminephrectomy causes the progression of glomerulosclerosis and apoptosis in high IgA strain ddY mice. Nephron. 2002;92:389–98.
Sato M, Nakajima Y, Koshikawa S. Effect of sodium cromoglycate on an experimental model of IgA nephropathy. Clin Nephrol. 1987;27:141–6.
Sadakane C, Hattori T, Koseki J, Inagaki Y, Hasegawa Y, Shindo S, et al. TJN-259 improves mesangial lesions in experimental immunoglobulin a nephropathy in ddY mice. Biol Pharm Bull. 2009;32(10):1728–33.
Chihara Y, Ono H, Ishimitsu T, Ono Y, Ishikawa K, Rakugi H, et al. Roles of TGF-beta1 and apoptosis in the progression of glomerulosclerosis in human IgA nephropathy. Clin Nephrol. 2006;65:385–92.
Nonaka Takahashi S, Fujita T, Takahashi T, Wada Y, Fuke Y, Satomura A, et al. TGF-beta1 and CTGF mRNAs are correlated with urinary protein level in IgA nephropathy. J Nephrol. 2008;21:53–63.
Oyama A, Muso E, Ono T, Matsushima H, Yashiro M, Suyama K, et al. Up-regulated TGF-beta mRNA expression in splenic T cells of high IgA-prone mice: a murine model of IgA nephropathy with glomerulosclerosis. Nephron. 2001;88:368–75.
Wang Y, Zhao MH, Zhang YK, Li XM, Wang HY. Binding capacity and pathophysiological effects of IgA1 from patients with IgA nephropathy on human glomerular mesangial cells. Clin Exp Immunol. 2004;136:168–75.
Hayashi K, Nagamatsu T, Ito M, Yagita H, Suzuki Y. Acteoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent (3): effect of aceteoside on expression of intercellular adhesion molecule-1 in experimental nephritic glomeruli in rats and cultured endothelial cells. Jpn J Pharmacol. 1996;70:157–68.
Hayashi K, Nagamatsu T, Ito M, Hattori T, Suzuki Y. Acetoside, a component of Stachys sieboldii MIQ, may be a promising antinephritic agent: effect of acteoside on crescentic-type anti-GBM nephritis in rats. Jpn J Pharmacol. 1994;65:143–51.
Hattori T, Fujitsuka N, Shindo S. Effect of acteoside on mesangial proliferation in rat anti-Thy 1 nephritis. Nippon Jinzo Gakkai Shi. 1996;38:202–12.
Hasegawa Y, Shindou S, Hattori T, Yamazaki Y, Obata T, Horiuchi F, et al. US Patent 2001; 6313153.
Muso E, Yoshida H, Takeuchi E, Shimada T, Yashiro M, Sugiyama T, Kawai C. Pathogenic role of polyclonal and polymeric IgA in a murine model of mesangial proliferative glomerulonephritis with IgA deposition. Clin Exp Immunol. 1991;84:459–65.
Muso E, Yoshida H, Takeuchi E, Yashiro M, Matsushima H, Oyama A, Suyama K, Kawamura T, Kamata T, Miyawaki S, Izui S, Sasayama S. Enhanced production of glomerular extracellular matrix in a new mouse strain of high serum IgA ddY mice. Kidney Int. 1996;50:1946–57.
Kagami S, Border WA, Ruoslahti E, Noble NA. Coordinated expression of TGF beta 1 integrins and transforming growth factor-beta-induced matrix proteins in glomerulonephritis. Lab Invest. 1993;69:68–76.
Marsh JB. Lipoprotein metabolism in experimental nephrosis. J Lipid Res. 1984;25:1619–23.
Daniel C, Wiede J, Krutzsch HC, Ribeiro SMF, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondine-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–68.
Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yoriola N, Lohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via MAPK and JNK: a mechanisms for activation of latent TGF-β1. Am J Physiol Renal Physiol. 2004;286:278–87.
Acknowledgments
The authors are grateful to Mr. Obata and Miss Horiuchi for synthesis of TJN-331.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Saegusa, Y., Sadakane, C., Koseki, J. et al. (E)-N-[(3,4-dimethoxyphenethyl)]-N-methyl-3-(3-pyridyl)-2-propenamide (TJN-331) inhibits mesangial expansion in experimental IgA nephropathy in ddY mice. Clin Exp Nephrol 14, 528–535 (2010). https://doi.org/10.1007/s10157-010-0338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-010-0338-4