Abstract
Tanaidaceans are small benthic crustaceans with a strictly benthic life cycle and low dispersion rates, so they are good candidates to evaluate the effects of environment over life history strategies and reproductive biology. In this work, we studied two populations of Tanais dulongii (Audouin, 1826) that live in two contrasting habitats in order to determine whether they differ in life history traits. The animals were obtained by systematic sampling in a rocky shore with a lower anthropic impact (La Estafeta: LE) and a polluted area (Mar del Plata harbour: MdP) from March 2011 to March 2012. Seawater temperature and salinity did not differ between sites, but MdP showed more acid and hypoxic conditions than LE. Population density was homogeneous and lower in MdP (ca. 20 ind/100 gr) than that in LE where density varied between 250 and 800 ind/100 gr. Reproductive individuals and juveniles were always present, and both populations showed two main recruitment periods: the first in spring in both populations, and the second in summer in MdP but in autumn–winter in LE. In both populations, sex ratio was strongly female-biased. Juveniles, females and males from LE had larger sizes than that from MdP and reached their sexual differentiation at larger sizes. The estimated lifespan was about 9 and 12 months in MdP and LE, respectively. This study suggests that the differences observed between populations of T. dulongii in life history traits are intimately related to environmental differences in pH and dissolved oxygen between habitats, but should not be discarded a synergistic effect of temperature, organic pollution, food availability and predation pressure.
Similar content being viewed by others
Introduction
The study of life history traits of a species is essential to understand its population biology and their ecological role (Stearns 2000). Life history theory predicts that organisms should assign resources to growth, survival and reproduction in such a way as to maximize their reproductive success (Stearns 2000). When environmental conditions change, altering the fitness or subsistence, organisms may change their patterns of resource allocation (e.g. shifting their reproductive strategy, growth rates and behaviour) in order to continue maximizing their reproductive success (Schaffer 1974; Winkler and Wallin 1987; Duffy and Thiel 2007). In consequence, it is expected that populations of the same species exposed to dissimilar environmental conditions differ in their life history traits (Stearns 2000).
Tanaidaceans are represented by almost 1200 species, mostly marine, and distributed from littoral to hadal zones (Błażewicz-Paszkowycz et al. 2012) where they are the main food source for many organisms (Nagelkerken and Van der Velde 2004; Agüero et al. 2014). Their reproductive strategies and characteristics vary between species (Schram 1986; Błażewicz-Paszkowycz et al. 2012). In general, tanaidaceans are dioecious and present a sexual dimorphism or polymorphism (Schram 1986; Dojiri and Sieg 1997). In reproductive periods, males migrate actively searching ovigerous females, which in some cases are free living or remain hidden in shelters (e.g. tubes or burrows), and lay her eggs in a ventral marsupium after copulation (Johnson and Attramadal 1982a; Mendoza 1982; Schram 1986). Once fertilization occurs, offspring pass through an abbreviated larval development (manca I and II) (Masunari 1983; Messing 1983; Hamers and Franke 2000). When mancae are released, they begin to develop independently near to the mother (Johnson and Attramadal 1982a). Due to their small size (in most cases, around 3 mm or less) and a strictly benthic life cycle with low dispersion rates, life history traits are intimately related to environmental issues (Schram 1986; Dojiri and Sieg 1997), and therefore, tanaidaceans might be good candidates to evaluate the effects on environment over life history strategies.
Tanais dulongii (Audouin 1826) is a tanaidacean species with a worldwide distribution, their type locality is the Mediterranean Sea off Egypt, but it has also been recorded as native species in North Europe (Andersson et al. 1978; Johnson and Attramadal 1982a; Holdich and Jones 1983; Fiser 2004; Bamber, 2012). On the other hand, the presence of T. dulongii in ballast water and ship fouling has favoured its invasion in numerous regions of the world (Hutchings et al. 1993, Rander et al. 2009; Bamber, 2012; Błażewicz-Paszkowycz et al. 2012), such as Australia (Hutchings et al. 1993), eastern North America (Sieg 1980) and the coasts of south-western South America where the status is considered cryptogenic (i.e. a likely introduced organism) (Orensanz et al. 2002). According to Poore (1996), this wide distribution is highly suspect due to some tanaidaceans are superficially similar favouring a taxonomic misidentification, so most researchers are convinced that T. dulongii is a species complex consisting of several species morphologically similar as has been shown for many other tanaidaceans (Bamber, 2012; Larsen et al., 2014). However, this assertion has not been verified yet due to the lack of genetic studies.
Tanais dulongii lives in self-constructed tubes and feeds principally on detritus and algae, and it is commonly found in benthic environments of Argentina (Adami et al. 2004; Sueiro et al. 2011; Rumbold et al. 2012, 2014). Several aspects of the biology of this species have been studied, such as behaviour, development, reproduction, morphometry and population dynamics (Johnson and Attramadal 1982a, b; Borowsky 1983; Perez-Ruzafa and Sanz 1993; Hamers and Franke 2000; Rumbold et al. 2012, 2014, 2015), but interpopulational comparative studies are scarce (Pennafirme and Soares-Gomes 2009; Rumbold et al. 2014). Recent works have established that populations of T. dulongii that live in two contrasting habitats differed in morphometry and relative growth patterns (Rumbold et al. 2014), but differences in reproductive aspects (e.g. sex ratio and fecundity), population dynamics and lifespan have not been studied yet.
The aim of this paper was to examine and compare the populational and reproductive biology between two populations of T. dulongii that live in two dissimilar habitats: a natural rocky shore and an artificial harbour area. The working hypothesis is that populations that live under different environmental conditions will differ in life history traits.
Materials and methods
Study area
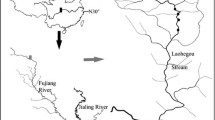
The study was conducted in Mar del Plata harbour (thereafter MdP, 38°02′29″S, 57°32′16″W; Fig. 1) and in La Estafeta (thereafter LE, 38°09′59″S, 57°37′59″W). MdP is one of the most important harbours in Argentina because of its fishing fleet, naval traffic, commercial trade and recreational sailing (Rivero et al. 2005; Schwindt et al. 2010; Albano et al. 2013). The coasts of Mar del Plata city (ca. 700,000 residents) are characterized by sandy beaches interrupted by several quartzite outcrops as Cabo Corrientes and Punta Mogotes (Isla and Lasta 2006; Schwindt et al. 2010). This harbour was built from 1913 to 1924 on the beach between these two outcrops (Isla and Lasta 2006; Schwindt et al. 2010). It consists of a semiclosed area limited by two artificial breakwaters made of concrete blocks and has a narrow mouth of about 300 m. Mean water depth is lower than 5 m, and a navigational channel is maintained with dredging (at 10 m). The bottom is composed by fine and very fine sand in the mouth, and silt in the inner harbour. MdP is a polluted area characterized by high levels of hydrocarbons from fuel discharges, polycyclic aromatic hydrocarbons, copper (DYOPSA 1999; Albano et al. 2013), tributyltin (Penchaszadeh et al. 2001; Goldberg et al. 2004), high water turbidity, low pH and high levels of organic matter from industrial and sewage effluents (Rivero et al. 2005; Schwindt et al. 2010; Albano et al. 2013). Despite to high pollution, in the recreational area, the wooden docks and marinas are covered by a high abundance of ascidians, algae and tubicolous polychaetes that generate refuges to a great variety of fish, flatworms, molluscs, crustaceans and nematodes (Albano and Obenat 2009; Albano et al. 2013). On the other hand, LE is an intertidal flat located 15 km south of MdP characterized by a rocky substrate formed by consolidated sediment and many tidal pools (Rumbold et al. 2012). The distance between cliffs and the water edge during the lowest tides is approximately 70 m. The rock surface is covered by algae (mainly Ulva rigida and Corallina officinalis) serving as sites for feeding, reproduction and shelter against to predation and drying to vertebrates and invertebrates (Rumbold et al. 2012). This environment is a natural habitat with lower anthropic impact, because surrounding cliffs make it difficult to access. Both sites are subjected to a microtidal regime with mean amplitude of 0.8 m (Isla 2004).
Field sampling and laboratory procedures
Samples were collected monthly from March 2011 to March 2012, except on June 2011 in LE due to bad weather conditions, and consist on five sampling units. The structure of both habitats is extremely different (two dimensions in LE, three dimensions in MdP), so a singly type of sampling unit was not possible. In LE, each sampling unit was defined by a 0.15 × 0.15 m quadrat placed over an algal patch; algae of this 0.0225 m2 quadrat were totally scraped up with spatulas. In MdP, each sampling unit was a mass of the fouling community adhered to docks extracted with a 830-cm3 core. Samples were fixed in situ in 98 % alcohol, and in the laboratory, they were sieved through a 0.35-mm mesh and weighed; organisms were sorted and counted using a stereomicroscope. Dissolved oxygen, pH and seawater temperature were measured in situ each time using a portable multiprobe instrument (HACH sensION 156), and salinity was measured with an optical refractometer.
Specimens of T. dulongii were sorted into five groups according to Almeida (1994) and Leite et al. (2003) on the basis of cheliped size and the presence of ovisacs (primordium of small brood sacs) or marsupia (developed brood sacs with eggs or embryos). The groups were males (with large chelipeds), pre-ovigerous females (with small chelipeds and ovisacs), ovigerous females (with small chelipeds and marsupium), post-ovigerous females (with small chelipeds but without ovisacs) and juveniles (all individuals measuring less than the smallest identifiable male, except those that had visible ovisacs). They were measured from the tip of the rostrum to the posterior edge of the pleotelson using a micrometric ocular on a stereoscopic microscope (total length, ±0.01 mm accuracy); the smallest identifiable male measured 2.81 mm in LE and 1.75 mm in MdP. Specimens were grouped in size classes of 0.25 mm, and size frequency distributions (SFD) were constructed for each population. Population density (individuals per 100 gr of sample) and sex ratio (males/(males + total females)) were calculated. The percentage of males and ovigerous females was used as an estimate of reproductive activity (Kneib 1992). Fecundity was estimated from 50 ovigerous females (with eggs at stage I) randomly selected; they were measured (total length), and their eggs removed from the marsupium and counted.
Data analysis
Parametric tests were used preferably, but when the assumptions of parametric statistics were seriously violated, an appropriate nonparametric test was applied (Underwood 1997). Statistical analyses were performed with the significance level set at P < 0.05.
To evaluate differences in environmental variables (factors: study sites and months), total density fluctuations (factors: study sites and months), variations in population groups (juveniles, males and females; factors: group and month) and to determine changes in female groups (pre-ovigerous, ovigerous and post-ovigerous; factors: group and month), a two-way ANOVA was used. A Student–Newman–Keuls (SNK) test was applied when statistically significant differences of means were found. A χ 2 test with Yates correction was applied to examine the deviation of sex ratio from an expected ratio of 1:1. Linear regression and determination coefficients were calculated for each population to assess the relationship between female size and the number of eggs in the marsupium; to determine the equality of regression lines, slopes and constants were tested by a Student’s t test. A Mann–Whitney U test was used to evaluate size differences in juveniles, females and males both between and within populations. Growth of T. dulongii was described by the seasonalized von Bertalanffy (VBGF) curve (see Bilgin et al. 2009 for details), and models were not fitted separately for each sex due to the low abundance of males in MdP. The growth curve was estimated by the Elefan I program (Pauly and David 1981).
Results
Environmental variables
Seawater temperature differed among months (two-way ANOVA, P < 0.05; Fig. 2a, Table 1), but not between sites (two-way ANOVA, P > 0.05). Annual mean temperature was ca. 16.5 °C, with highest temperatures in January 2012 (ca. 24 °C) and the lowest in August 2011 (ca. 9 °C). Salinity did not differ between sites and months (two-way ANOVA, P > 0.05; Fig. 2b). Annual mean salinity was 33.92 ± 0.29 PSU in LE and 32.17 ± 3.24 PSU in MdP. However, during December 2011, salinity reached an extraordinary low value in MdP (ca. 22 PSU). On the other hand, pH and dissolved oxygen (DO) were always lower in MdP than those in LE (pH: 8.23 ± 0.14, DO: 8.71 ± 1.42 mg/l in MdP, pH: 8.55 ± 0.22, DO: 12.69 ± 1.65 mg/l in LE; two-way ANOVA, P < 0.05; Fig. 2c, d).
Population structure
Density of T. dulongii differed among study sites and months sampled (two-way ANOVA, P < 0.001; Table 2). It was similar in MdP during the whole sampling period (17 ± 6 ind/100 gr; SNK test, P > 0.05) but in LE it remained below 250 ind/100 gr on March 2011 and from August to January 2012 (SNK test, P > 0.05), and reached high values from April to July 2011 and on March 2012 (between 350 and 800 ind/100 gr; SNK test, P < 0.05).
Density of males, females and juveniles differed among groups and months, in both populations (two-way ANOVA, P < 0.01). It was similar from March 2011 to January 2012 (ca. 10 ind/100 gr; SNK test, P > 0.05) in MdP, except for March 2012 in which female density was higher than that of other groups, reaching values of ca. 90 ind/100 gr (SNK test, P < 0.001; Fig. 3a). Density of males remained similar in LE during the sampling period (11 ind/100 gr; SNK test, P > 0.05), but females and juveniles had always higher values (ca. 50 ind/100 gr; SNK test, P > 0.05), reaching their maximum from April to July 2011 and March 2012 (ca. 200–450 ind/100 gr; SNK test, P < 0.05; Fig. 3b).
Density of pre-ovigerous, ovigerous and post-ovigerous females varied among groups and months in both populations (two-way ANOVA, P < 0.05). It did not differ significantly between groups in MdP (2–10 ind/100 gr; SNK test, P > 0.05; Fig. 4a) from March 2011 to January 2012, but in March 2012 post-ovigerous females were more abundant (ca. 70 ind/100 gr; SNK test, P < 0.05). Pre-ovigerous and ovigerous females had the lowest densities in La Estafeta but did not differ between months (ca. 15 and 35 ind/100 gr, respectively; SNK test, P > 0.05; Fig. 4b); post-ovigerous females had values of ca. 20–70 ind/100 gr during most months sampled, but reached their maximum values from April to July 2011 (SNK test, P > 0.05), September 2011 and March 2012 (ca. 100–300 ind/100 gr; SNK test, P < 0.05).
The mean annual proportion of ovigerous females with respect to total females was 9.55 % in MdP. The maximum values of this proportion (>13 %) appeared in December 2011 and January 2012, and ovigerous females were absent during March, September and October 2011. On the contrary, ovigerous females were always present in LE with a mean annual proportion of 10.14 %, maximum values in November and December (>20 %) and minimum values in May and July (1.11–2.52 %).
Sex ratio, reproductive activity and fecundity
The mean sex ratio was 0.06 ± 0.01 and 0.08 ± 0.02 in MdP and LE, respectively, and differed significantly from the expected 1:1 (χ 2 test, P < 0.05). The monthly sex ratio was always female-biased in both environments and varied throughout the study period (χ 2 test, P < 0.05). The highest value in MdP was recorded on December 2011 (0.27; χ 2 test, P < 0.05; Fig. 5a), and the minimum on May, July and September (0.00; χ 2 test, P < 0.05). Sex ratio remained between 0.8 and 0.14 in LE during the sampled period (χ 2 test, P < 0.05; Fig. 5b), reaching the lowest values on April, May and January (<0.02; χ 2 test, P < 0.05). The proportions of males and ovigerous females were lower than 25 % in MdP and LE (except for males in October 2011 in MdP).
Fecundity increased with female size in both populations (MdP: r 2 = 0.74, P < 0.001; LE: r 2 = 0.72, P < 0.001; Fig. 6). Constants (a) and slopes (b) of regression lines differed among populations (in both cases, Student’s t test, P < 0.001): females of LE exhibited a higher increase in the relationship between size and number of eggs in respect of females from MdP. In addition, the mean number of eggs observed (±standard deviation) per female differed between sites (Student’s t test, P < 0.001), being higher in LE (39.1 ± 15.3) than in MdP (17.8 ± 12.1).
Length-frequency analysis
Juveniles, females and males from LE showed larger sizes than that from MdP (in all cases, Mann–Whitney U test, P < 0.001; Fig. 7). Females presented larger size than males in MdP, and the opposite occurred in LE (in both cases, Mann–Whitney U test, P < 0.001). Furthermore, despite mean size was slightly different between sexes (<0.18 mm) in both populations, females reached the maximum sizes exceeding males’ size by more than 1.5 mm. The smallest differentiated female measured 1.19 and 2.19 mm in MdP and LE, respectively. Ovigerous females were smaller in MdP than those in LE, and only some ovigerous females of MdP reached similar sizes as in LE (Mann–Whitney U test, P < 0.001).
The SFD analysis allowed identifying two cohorts in MdP (Fig. 8); the first corresponded to large individuals present from March 2011 to January 2012, and the second included juveniles recruited in November 2012 that increased their size until March 2012. Three cohorts were identified in LE, and two of them coexisted: the first cohort, composed by juveniles and adults, was present from March 2011 to March 2012, and the second, formed by large-size individuals, was detected from March 2011 to May 2011, and the third included only juveniles and was observed from September 2011 to March 2012. The growth parameters obtained by Elefan I showed that individuals from LE reached a L∞ (8.00 mm) higher than individuals from MdP (7.00 mm), while the K coefficient was lower in LE (0.55 year−1) than that in MdP (0.63 year−1).
Discussion
Environmental conditions showed that water has the same seasonal variation of temperature in both habitats, but it was more acid and hypoxic in MdP than in LE as previously reported (Rivero et al. 2005; Schwindt et al. 2010; Albano et al. 2013). No differences were observed in salinity between habitats except the extraordinary low value recorded in December in MdP (related to heavy rainfall; Martos et al. 2004); that fact suggests short-term variations of salinity in this site. Consequently, T. dulongii should face a priori more stressful conditions in MdP than those in LE, which would explain some differences observed in life history traits between populations, as reported before (Rumbold et al. 2014) and in this study.
A close relationship between density and temperature, characterized by the lowest values in warmer months and the highest in colder months, was observed in LE, but not in MdP with a lower but constant density through the year. The pattern observed in LE has been reported in other tanaidaceans (Mendoza 1982; Masunari 1983; Kneib 1992). Temperature plays a major role in intertidal environments: during the warm season, higher temperatures increase mortality rates of benthic species by desiccation, while in cold season lower temperatures reduce the desiccation and consequently mortality (Kneib 1984; Bertness 1999). This would explain not only the changes in abundance in LE, but also, in conjunction with the high reproductive activity recorded in previous months, the main recruitment registered in autumn and winter. Other factors may explain the low density recorded in MdP during the study period: it was demonstrated that larval and juvenile stages have high mortality rates related to adverse environmental conditions, such as sudden changes in salinity, low pH and dissolved oxygen, and high concentrations of organic matter (Stoner 1986; Chintiroglou et al. 2004; Kalkan et al. 2007; de la Ossa Carretero et al. 2010). Moreover, temperature influences reproduction of other peracarids as amphipods, isopods and mysids: higher temperatures promote juvenile growth and sexual maturation (Pöckl 1992; McKenney and Celestial 1995; Maranhão and Marques 2003; Fockedey et al. 2005; Tsoi et al. 2005; Henninger et al. 2010; Hosono 2011). A similar pattern has also been reported in populations of T. dulongii from Norway and Spain (Johnson and Attramadal 1982a; Perez-Ruzafa and Sanz 1993) and may explain the higher reproductive activity recorded during the warmer months in both populations.
Egg number was linearly related to female size in both populations and was similar to those reported in other tanaidacean species by Larsen (2005) but was higher than the values registered by other authors (Masunari and Sieg 1980; Masunari 1983; Messing 1983; Schmidt et al. 2002). However, the mean number of eggs per female and the slopes of fecundity regressions from MdP were lower than those from LE. As mentioned above, the stressful conditions of an environment may involve the shifting of resources from reproduction to survival (Stearns 2000), which would explain the observed variations in fecundity among populations. In peracarids, fecundity can be affected by many factors, such as pollutants, food availability, latitude, salinity and temperature (Corey 1981; France 1992; Maranhão and Marques 2003; Ford et al. 2004; Pennafirme and Soares-Gommes 2009). The results of this study are inconclusive to determine whether there is an effect of the environment on fecundity due to the difference in size range of ovigerous females of both populations.
Tanais dulongii had smaller sizes and reached sexual maturity earlier in MdP than in LE, as has been previously reported (Rumbold et al. 2014), but a higher growth rate and a shorter lifespan were found in the former site in this study. Similar differences between polluted and pristine environments were detected in the tanaidacean Apseudopsis latreillii (Milne-Edwards 1828) (de la Ossa Carretero et al. 2010). Several factors that affect growth and size characterize polluted sites as MDP: low oxygen content, low pH and high organic matter content. Oxygen availability influences size among and within amphipod species (e.g. Nebeker et al. 1992; Chapelle and Peck 2004). Low pH, synergically with low salinity, retarded the embryonic development of Echinogammarus marinus (Leach 1815) and later affected life history traits such as growth, maturity and reproduction (Egilsdottir et al. 2009). Moreover, environmental factors related to very nature of the habitat should not be discarded, such as food availability and predation pressure (Stearns 2000). Food resources are one of the main factors involved in growth and sexual maturation of crustaceans (Wenner et al. 1974; Hines 1989; Moore and Farrar 1996; Castiglioni et al. 2007; Cuzon et al. 2008). However, a higher organic matter content could favour maturity at smaller sizes, as occur in several peracarid populations where under stressful conditions invest more energy in reproduction than growth (Clarke 1987; Lozoya and Defeo 2006). On the other hand, it has been suggested that in populations of other crustaceans (e.g. crabs, shrimps and amphipods) that reach adulthood at relatively smaller sizes, predation pressure is higher on larger organisms, allowing to reach maturity at younger age, and consequently at smaller size, and to reproduce before being killed. This hypothesis implies that natural factors may explain the increase in the presence of this phenotype (small size) in the population (Wellborn 1994; Schlining and Spratt 2000; Zhang et al. 2004). Unfortunately, the effects of pollutants, environmental variables on development of T. dulongii and data of potential predators in both environments are lacking, so the explanation of the proximal causes of the observed differences would require more studies and detailed laboratory and field experiments.
In both populations, the maximum size corresponded to females (Rumbold et al. 2014; this study). Differences in body sizes between sexes should be related to the fact that the development is more complex and compromise more instars in females than in males (Hamers and Franke 2000). Furthermore, as mentioned above, larger sizes would favour an increase in fecundity and fitness in females (Rumbold et al. 2012), while smaller sizes in males may provide them a greater mobility, increasing the number of mates and reducing the risks of predation (Johnson and Attramadal 1982b; Borowsky 1983; Kakui 2015).
Two main recruitment periods were observed in T. dulongii, as occurs in Monokalliapseudes schubarti (Mañé-Garzón 1949), another intertidal tanaidacean of south-western Atlantic (Fonseca and D’Incao 2003; Leite et al. 2003) and in other benthic invertebrates of the study area, such as the amphipods Monocorophium acherusicum and Melita palmata, the decapod Cyrtograpsus angulatus and the polychaete Ficopomatus enigmaticus (Gavio 2003; Obenat 2002; Obenat et al. 2006; Ruíz Barlett 2012). Individuals recruited in late spring grew quickly in MdP and matured at reduced sizes in midsummer, while in LE juveniles recruited in spring grew more slowly and matured in late summer at larger sizes. Later, individuals of both populations continued growing and reproducing during autumn and winter until they disappeared in early or mid-spring when maximum sizes of adults and a decrease in density were observed, suggesting a high mortality of larger individuals. However, a second group of individuals that recruited in summer in MdP and autumn in LE could survive the colder months and growth in the following spring and even summer, reaching the largest sizes in both populations. Although both populations showed their first recruitment during spring, the second one differed between them, and this could be explained by the observed differences in growth rates. The estimated lifespan was about 9 and 12 months in MdP and LE, respectively. Moreover, SFD showed that MdP population is less structured than LE. This fact, combined with the abrupt increase of juveniles and females in MdP during January and March and the fact that ovigerous females were always present in LE but not in MdP, would suggest that MdP acts as a sink of recruits and possibly females from natural habitats. However, the small size of adults and the absence of planktonic stages in T. dulongii (Johnson and Attramadal 1982a, b; Hamers and Franke 2000), the presence of soft-bottom beaches on the sides of the harbour that grant certain isolation from nearby natural and artificial environments and the strong littoral current in the harbour entrance (Isla 2004; Martos et al. 2004; Rivero et al. 2005) would make very difficult the arrival of individuals from LE.
Finally, the presence of T. dulongii in MdP throughout the study period and their low density suggest that this species is tolerant to organic pollution and physical disturbance, confirming the idea of Kalkan et al. (2007). In addition, Ward and Hutchings (1996) found a population of T. dulongii in environments with high concentrations of heavy metals. However, studies related to the effects of pollutants on this species are lacking (Kalkan et al. 2007). This study together with future bio-assays (e.g. Ambrosio et al. 2014) could reveal the potential of T. dulongii as a bio-indicator species to assess the health status of marine environments.
The present study suggests that the differences observed between populations of T. dulongii, in life history traits, such as density, recruitment period, sizes and growth rates are intimately related to environmental differences between habitats. Further research is necessary to elucidate if population dynamics, reproductive aspects (present study) and morphometric differences (Rumbold et al. 2014) could be related with genetic and reproductive isolation between populations.
References
Adami ML, Tablado A, Gappa JL (2004) Spatial and temporal variability in intertidal assemblages dominated by the mussel Brachidontes rodriguezii (d’Orbigny, 1846). Hydrobiologia 520:49–59
Agüero ML, García-Borboroglu P, Esler D (2014) Trophic ecology of breeding white-headed steamer-duck (Tachyeres leucocephalus). Waterbirds 37:88–93
Albano MJ, Obenat SM (2009) Assemblage of benthic macrofauna in the aggregates of the tubiculous worm Phyllochaetopterus socialis in the Mar del Plata harbour, Argentina. J Mar Biol Assoc UK 89:1099–1108
Albano MJ, Lana P, Bremec C, Elías R, Martins C, Venturini N, Muniz P, Rivero S, Vallarino EA, Obenat S (2013) Macrobenthos and multi-molecular markers as indicators of environmental contamination in a South American port (Mar del Plata, Southwest Atlantic). Mar Pollut Bull 73:102–114
Almeida MVO (1994) Kalliapseudes schubarti Mañé-Garzon, 1949 (Tanaidacea-Crustacea): dinâmica populacional e interações com a macrofauna bêntica no Saco do Limoeiro, Ilha do Mel (Paraná, Brasil). Master thesis, Universidade Federal do Paraná, Brasil
Ambrosio ES, Ferreira AC, Capítulo AR (2014) The potential use of Sinelobus stanfordi (Richardson, 1901) (Crustacea, Tanaidacea) as a biological indicator of water quality in a temperate estuary of South America. Limnetica 33:139–152
Andersson A, Hallberg E, Johnson SB (1978) The fine structure of the compound eye of Tanais cavolinii Milne-Edwards (Crustacea: Tanaidacea). Acta Zool (Stockholm) 59:49–55
Bamber RM (2012) Littoral Tanaidacea (Crustacea: Peracarida) from Macaronesia: allopatry and provenance in recent habitats. J Mar Biol Assoc UK 92:1095–1116
Bertness MD (1999) The ecology of Atlantic shorelines. Sinauer Associates, Sunderland
Bilgin S, Samsun O, Ozen O (2009) Seasonal growth and reproduction biology of the Baltic prawn, Palaemon adspersus (Decapoda: Palaemonidae), in the southern Black Sea. J Mar Biol Assoc UK 89:509–519
Dojiri M, Sieg J (1997) The Tanaidacea. In: Blake JA, Scott PH (eds) Taxonomic atlas of the benthic fauna of the Santa Maria Basin and Western Santa Barbara Channel. Volume 11 - The crustacea part 2: the Isopoda, Cumacea and Tanaidacea, 1st edn. Santa Barbara Museum of Natural History, Santa Barbara, pp 181–268
Błażewicz-Paszkowycz M, Bamber R, Anderson G (2012) Diversity of Tanaidacea (Crustacea: Peracarida) in the world’s oceans—How far have we come? PLoS ONE 7:e33068-11. doi:10.1371/journal.pone.0033068
Borowsky B (1983) Reproductive behavior of three tube-building peracarid crustaceans: the amphipods Jassa falcata and Amphitoe valida and the tanaid Tanais cavolinii. Mar Biol 77:257–263
Castiglioni DS, Garcia-Schroeder D, Ferreira Barcelos D, Bond-Buckup G (2007) Intermolt duration and postembryonic growth of two sympatric species of Hyalella (Amphipoda, Dogielinotidae) in laboratory conditions. Nauplius 15:57–64
Chapelle G, Peck LS (2004) Amphipod crustacean size spectra: new insights in the relationships between size and oxygen. Oikos 106:167–175
Chintiroglou CC, Antoniadou C, Baxevanis A, Damianidis P, Karalis P, Vafidis D (2004) Peracarida populations of hard substrate assemblages in ports of the NW Aegean Sea (eastern Mediterranean). Helgoland Mar Res 58:54–61
Clarke A (1987) Temperature, latitude and reproductive effort. Mar Ecol Prog Ser 38:89–99
Corey S (1981) Comparative fecundity and reproductive strategies in seventeen species of the Cumacea (Crustacea: Peracarida). Mar Biol 62:65–72
Cuzon G, Gaxiola G, Rosas C (2008) Nutrition in relation to reproduction in crustaceans. In: Mente E (ed) Reproductive biology crustaceans, 1st edn. Science Publishers, Einfield, pp 319–364
de la Ossa Carretero JA, del Pilar Ruso Y, Giménez Casalduero F, Sánchez Lizaso JL (2010) Sensitivity of tanaid Apseudes latreillei (Milne-Edwards) populations to sewage pollution. Mar Environ Res 69:309–317
Duffy JE, Thiel M (2007) Evolutionary ecology of social and sexual systems: crustaceans as model organisms. Oxford University Press, New York
DYOPSA S. Dredging International (1999) Estudio de dispersión de sedimentos dragados y contaminantes para la obra de dragado del Puerto de Mar del Plata. Informe Final. Serman & Asociados SA Consulting Company, Philippines
Egilsdottir H, Spicer JI, Rundle SD (2009) The effect of CO2 acidified sea water and reduced salinity on aspects of the embryonic development of the amphipod Echinogammarus marinus (Leach). Mar Pollut Bull 58:1187–1191
Fiser C (2004) Prispevek k poznavanju skarjevk (Tanaidacea: Peracarida: Crustacea) v slovenskem morju. Nat Slov 6:11–17
Fockedey N, Meesa J, Vangheluwe M, Verslycked T, Janssen CR, Vincxa M (2005) Temperature and salinity effects on post-marsupial growth of Neomysis integer (Crustacea: Mysidacea). J Exp Mar Biol Ecol 326:27–47
Fonseca DB, D’Incao F (2003) Growth and reproductive parameters of Kalliapseudes schubartii in the estuarine region of Lagoa dos Patos (southern Brazil). J Mar Biol Assoc UK 83:931–935
Ford AT, Fernandes TF, Read PA, Robinson CD, Davies IM (2004) The costs of intersexuality: a crustacean perspective. Mar Biol 145:951–957
France RL (1992) Biogeographical variation in size-specific fecundity of the amphipod Hyalella azteca. Crustaceana 62:240–248
Gavio MA (2003) Hábitat, sistemas de apareamiento y selección sexual en dos especies simpátricas de Cyrtograpsus (Decapoda: Brachyura: Grapsidae). Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina
Goldberg RN, Averbuj A, Cledón M, Luzzatto D, Sbarbati Nudelman N (2004) Search for triorganotins along the Mar del Plata (Argentina) marine coast: finding of tributyltin in egg capsules of a snail Adelomelon brasiliana (Lamarck, 1822) population showing imposex effects. Appl Organomet Chem 18:117–123
Hamers C, Franke HD (2000) The postmarsupial development of Tanais dulongii (Audouin, 1826) (Crustacea, Tanaidacea) in laboratory culture. Sarsia 85:403–410
Henninger TO, Froneman PW, Booth AJ, Hodgson AN (2010) Growth and longevity of Exosphaeroma hylocoetes (Isopoda) under varying conditions of salinity and temperature. J Afr Zool 45:41–51
Hines AH (1989) Geographic variation in size at maturity in brachyuran crabs. Bull Mar Sci 45:356–368
Holdich DM, Jones JA (1983) The distribution and ecology of British shallow-water tanaid crustaceans (Peracarida, Tanaidacea). J Nat His 17:157–183
Hosono T (2011) Effect of temperature on growth and maturation pattern of Caprella mutica (Crustacea, Amphipoda): does the temperature-size rule function in caprellids? Mar Biol 158:363–370
Hutchings PA, Ward TJ, Waterhouse J, Walker L (1993) Infauna of marine sediments and seagrass beds of Upper Spencer Gulf near Port Pirie, South Australia. Trans R Soc South Aust 117:1–15
Isla FI (2004) Geología del sudeste de Buenos Aires. In: Boschi EE, Cousseau MB (eds) La vida entre mareas: vegetales y animales de las costas de Mar del Plata, Argentina, 1st edn. Publicaciones Especiales INIDEP, Mar del Plata, pp 19–28
Isla FI, Lasta CA (2006) Manual de manejo costero para la Provincia de Buenos Aires. EUDEM, Mar del Plata
Johnson SB, Attramadal YG (1982a) Reproductive behaviour and larval development of Tanais cavolinii (Crustacea: Tanaidacea). Mar Biol 71:11–16
Johnson SB, Attramadal YG (1982b) A functional-morphological model of Tanais cavolinii Milne-Edwards (Crustacea, Tanaidacea) adapted to a tubicolous life-strategy. Sarsia 67:29–42
Kakui K (2015) First report of Zeuxo sp. (Crustacea: Tanaidacea) as prey for the forktongue goby, Chaenogobius annularis Gill, 1859. Rishiri Stud 34:1–6
Kalkan E, Karhan SÜ, Mutlu E, Simboura N, Bekbölet M (2007) Application of the bentix index in assessing ecological quality of hard substrata: a case study from the Bosphorus Strait, Turkey. Mediterr Mar Sci 8:15–29
Kneib RT (1984) Patterns of invertebrate distribution and abundance in the intertidal salt marsh: causes and questions. Estuaries 7:392–412
Kneib RT (1992) Population dynamics of the tanaid Hargeria rapax (Crustacea: Peracarida) in a tidal marsh. Mar Biol 113:437–445
Larsen K (2005) Deep-sea Tanaidacea (Peracarida) from the Gulf of Mexico. Brill, Leiden
Larsen K, Tuya F, Froufe E (2014) Genetic divergence of tanaidaceans (Crustacea: Peracarida) with low dispersal ability. Sci Mar 78:81–90
Leite FPP, Turra A, Souza ECF (2003) Population biology and distribution of the tanaid Kalliapseudes schubarti Mañé-Garzon, 1949, in an intertidal flat in southeastern Brazil. Braz J Biol 63:469–479
Lozoya JP, Defeo O (2006) Effects of a freshwater canal discharge on an ovoviviparous isopod inhabiting an exposed sandy beach. Mar Freshwater Res 57:421–428
Maranhão P, Marques JC (2003) The influence of temperature and salinity on the duration of embryonic development, fecundity and growth of the amphipod Echinogammarus marinus Leach (Gammaridae). Acta Oecol 24:5–13
Martos P, Reta R, Guerrero RA (2004) El ambiente físico de las costas marplatenses: su clima y sus aguas. In: Boschi EE, Cousseau MB (eds) La vida entre mareas: vegetales y animales de las costas de Mar del Plata, Argentina, 1st edn. Publicaciones Especiales INIDEP, Mar del Plata, pp 29–42
Masunari S (1983) Postmarsupial development and population dynamics of Leptochelia savignyi (Krøyer, 1842) (Tanaidacea). Crustaceana 44:151–162
Masunari S, Sieg J (1980) Morphological and ecological notes on Zeuxo coralensis Sieg, 1980 from Brazil. Stud Neotrop Fauna E 15:1–8
McKenney CL, Celestial DM (1995) Interactions among salinity, temperature, and growth of the estuarine mysid Mysidopsis bahia reared in the laboratory in the complete life cycle. 1. Body mass and age specific growth rate. J Crustacean Biol 15:169–178
Mendoza JA (1982) Some aspects of the autecology of Leptochelia dubia (Krøyer, 1842) (Tanaidacea). Crustaceana 43:225–240
Messing CG (1983) Postmarsupial development and growth of Pagurapseudes largoensis McSweeny (Crustacea, Tanaidacea). J Crustacean Biol 3:380–408
Moore DW, Farrar JD (1996) Effect of growth on reproduction in the freshwater amphipod, Hyalella azteca (Saussure). Hydrobiologia 328:127–134
Nagelkerken I, Van der Velde G (2004) Relative importance of interlinked mangroves and seagrass beds as feeding habitats for juvenile reef fish on a Caribbean island. Mar Ecol Prog Ser 274:153–159
Nebeker AV, Onjukka ST, Stevens DG, Chapman GA, Dominguez SE (1992) Effects of low dissolved oxygen on survival, growth and reproduction of Daphnia, Hyalella and Gammarus. Environ Toxicol Chem 11:373–379
Obenat S (2002) Estudios ecológicos de Ficopomatus enigmaticus (Polychaeta: Serpulidae) en la laguna Mar Chiquita, Buenos Aires, Argentina. Ph.D. Thesis, Universidad Nacional de Mar del Plata, Mar del Plata, Argentina
Obenat S, Spivak E, Garrido L (2006) Life history and reproductive biology of the invasive amphipod Melita palmata (Amphipoda: Melitidae) in the Mar Chiquita coastal lagoon, Argentina. J Mar Biol Assoc UK 86:1381–1387
Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, Darrigan G, Elías R, Lopez Gappa JJ, Obenat S, Pascual M, Penchaszadeh P, Piriz ML, Scarabino F, Spivak ED, Vallarino EA (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biol Invasions 4:115–143
Pauly D, David N (1981) ELEFAN I, a basic program for the objective extraction of growth parameters from length-frequency data. Meeresforschung 28:205–211
Penchaszadeh PE, Averbuj A, Cledón M (2001) Imposex in Gastropods from Argentina (south-western Atlantic). Mar Pollut Bull 42:790–791
Pennafirme S, Soares-Gomes A (2009) Population biology and reproduction of Kalliapseudes schubartii Mañé-Garzón, 1949 (Peracarida, Tanaidacea) in a tropical coastal Lagoon, Itaipu, southeastern Brazil. Crustaceana 82:1509–1526
Perez-Ruzafa A, Sanz MC (1993) Tipificación de las poblaciones de dos especies de tanaidáceos del Mar Menor (Murcia, SE de España). Publ Espec Inst Esp Oceanogr 11:159–167
Pöckl M (1992) Effects of temperature, age and body size on moulting and growth in the freshwater amphipods Gammarus fossarum and G. roeseli. Freshwater Biol 27:211–225
Poore GCB (1996) Species differentiation in Synidotea (Isopoda: Idoteidae) and recognition of introduced marine species: a reply to Chapman and Carlton. J Crustacean Biol 16:384–394
Rander A, Mayer S, Grabow K, Martens A (2009) Die Scherenassel Tanais dulongii (Audouin) an Bennenfrachtschiffen im Oberrhein (Crustacea: Tanaidacea). Lauterbornia 67:47–51
Rivero MS, Elías R, Vallarino EA (2005) First survey of macroinfauna in the Mar del Plata Harbor (Argentina), and the use of polychaetes as pollution indicators. Rev Biol Mar Oceanogr 40:101–108
Ruíz Barlett T (2012) Estructura, abundancia y distribución de las poblaciones nativas e invasoras de anfípodos en el puerto de Mar del Plata, provincia de Buenos Aires, Argentina. Tesis de Licenciatura, Universidad Nacional de Mar del Plata, Mar del Plata, Argentina
Rumbold CE, Obenat SM, Spivak ED (2012) Life history of Tanais dulongii (Tanaidacea: Tanaidae) in an intertidal flat in the southwestern Atlantic. J Crustacean Biol 32:891–898
Rumbold CE, Obenat SM, Spivak ED (2014) Morphometry and relative growth of populations of Tanais dulongii (Audoin, 1826) (Tanaidacea: Tanaidae) in pristine and impacted marine environments of the southwestern Atlantic. J Crustacean Biol 34:581–592
Rumbold CE, Obenat SM, Leonardi MS, Spivak ED (2015) Intersex in the gonochoristic crustacean Tanais dulongii (Audouin, 1826) (Peracarida: Tanaidacea: Tanaididae): a comparison of external reproductive characteristics. J Nat His 49:775–788
Schaffer WM (1974) Selection for optimal life histories: the effects of age structure. Ecology 55:291–303
Schlining KL, Spratt JD (2000) Assessment of Carmel Bay spot prawn, Pandalus platyceros, resource and trap fishery adjacent to an ecological reserve in central California. Crustac Issues 12:751–762
Schmidt A, Siegel V, Brandt A (2002) Postembryonic development of Apseudes heroae and Allotanais hirsutus (Tanaidacea, Crustacea) in Magellanic and sub-Antarctic waters. Antarct Sci 14:201–211
Schram FR (1986) Crustacea. Oxford University Press, New York
Schwindt E, Darrigan G, Repizo H (2010) Evaluación nacional de situación en materia del agua de lastre en el litoral marino y fluvial, Argentina. Informe Final. Proyecto Globallast
Sieg J (1980) Taxonomische monographie der Tanaidae Dana 1849 (Crustacea: Tanaidacea). Abh Senckenb Nat Gesell 537:1–267
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Stoner AW (1986) Cohabitation on algal habitat islands by two hermaphroditic Tanaidacea (Crustacea: Peracarida). J Crustacean Biol 6:719–728
Sueiro MC, Bortolus A, Schwindt E (2011) Habitat complexity and community composition: relationships between different ecosystem engineers and the associated macroinvertebrate assemblages. Helgoland Mar Res 65:467–477
Tsoi KH, Chiu KM, Chu KH (2005) Effects of temperature and salinity on survival and growth of the amphipod Hyale crassicornis (Gammaridea, Hyalidae). J Nat His 39:325–336
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Ward TJ, Hutchings PA (1996) Effects of trace metals on infaunal species composition in polluted intertidal and subtidal marine sediments near a lead smelter, Spencer Gulf, South Australia. Mar Ecol Prog Ser 135:123–135
Wellborn GA (1994) The mechanistic basis of body size differences between two Hyalella (amphipoda) species. J Freshw Ecol 9:159–168
Wenner AM, Fusaro C, Oaten A (1974) Size at onset of sexual maturity and growth rate in crustacean populations. Can J Zool 52:1095–1106
Winkler DW, Wallin K (1987) Offspring size and number—a life-history model linking effort per offspring and total effort. Am Nat 129:708–720
Zhang Z, Hajas W, Phillips A, Boutillier JA (2004) Use of length-based models to estimate biological parameters and conduct yield analysis for male Dugeness crab (Cancer magister). Can J Fish Aquat Sci 61:2126–2134
Acknowledgments
The authors would like to thank to Club Náutico de Mar del Plata to facilitate the access to the harbour. This paper was funded by Grants from the Universidad Nacional de Mar del Plata (EXA 610/12, EXA705/14) and from the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (CONICET) (PIP 112-201101-00830). C.E. Rumbold has a graduate fellowship from CONICET, and the results of this study will be part of his doctoral dissertation. Three anonymous referees provided useful comments which contributed to improve the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke.
Rights and permissions
About this article
Cite this article
Rumbold, C.E., Obenat, S.M. & Spivak, E.D. Comparison of life history traits of Tanais dulongii (Tanaidacea: Tanaididae) in natural and artificial marine environments of the south-western Atlantic. Helgol Mar Res 69, 231–242 (2015). https://doi.org/10.1007/s10152-015-0432-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-015-0432-9