Abstract
During spring and summer 2003, we measured a variety of chemical and biological parameters in five medium-sized, Mediterranean cage farms that exploit semi-offshore conditions, and controlled the supply of fodder. The objective was to assess whether modern cage farms proliferating at semi-offshore sites exert environmental impact levels equivalent to the levels described from more traditional cage farms located in shallow, sheltered sites. In the water column, we examined the concentration of dissolved inorganic nutrients and heterotrophic bacteria in both surface and near-bottom water. At the bottom, we examined the concentrations of benthic chlorophyll a, phaeophytin and organic matter in sediments, the granulometric structure of the sediment, and the taxonomic (at the family level) abundance of benthic macroinvertebrates. For most parameters, we found no substantial differences between farm and control sites. Rather, most variation was explained as a function of depth (surface versus bottom water) or season (spring versus summer conditions). Deviations of farm values from control values, when they occurred, were small and did not indicate any significant impact on either bacterioplankton or benthic chlorophyll. Only one of the five farms studied exerted a detectable impact on the benthic macroinvertebrate community immediately under the cages. These results suggest that medium-sized fish farms located on semi-exposed western Mediterranean coasts have fewer environmental impacts than traditional fish farms located in shallow, sheltered sites. Impact characterization in these new farms may require refinement of the standard approach to deal with rapid dispersal of effluents and sub-lethal levels of environmental disturbance.
Similar content being viewed by others
Introduction
Since floating cages for intensive salmon aquaculture started proliferating in North Atlantic waters in the early 1980s, marine ecologists have been concerned with the environmental impact of these installations. Studies on pioneering salmon farms located in fjords, lochs, and semi-enclosed bays on the Atlantic coasts of Europe and North America, and in the Pacific, soon revealed that cage farms generate both particulate organic wastes and soluble inorganic wastes (e.g., Brown et al. 1987; Ritz et al. 1990; Black 1991; Persson 1991; Gowen and Ezzi 1992; Hargrave et al. 1993; Gowen 1994; Ackefors et al. 1994; Findlay and Watling 1994; GESAMP 1996). Particulate organic materials (mostly fecal material and uneaten fodder) settle to the sea floor, forming dark sediments characterized by high levels of organic matter, nitrogen, and phosphorous, and reduced sulfur compounds. This sediment is a suitable substratum for bacterial growth, which in extreme cases induces severe oxygen depletion in sediment and bottom waters (e.g., Tsutsumi and Kikuchi 1983; Brown et al. 1987). In addition, sustained input of phosphorous and several nitrogen compounds from farming installations may alter natural concentration ratios of basic nutrients in the water column at the local scale (e.g., Gowen and Ezzi 1992), sometimes favoring local algal blooms and eutrophication processes (e.g., Persson 1991).
It has recently been suggested that Mediterranean sea bass and sea bream farms have a somewhat lesser environmental impact than Atlantic salmon farms (Karakassis 2001). Such a suggestion is based on data obtained from a limited number of farms, which in most cases are located in the eastern-Mediterranean basin (e.g., Karakassis et al. 1998, 1999, 2000, 2001; Pitta et al. 1999) because farming of finfish species has traditionally been dominated by Egypt, Greece, and Turkey. Similar to Atlantic and Pacific salmon farms, most of these eastern-Mediterranean sea bass and sea bream farms are located at shallow sheltered sites and semi-enclosed bays, a location choice favored by the complex coastal structure of Greece, Croatia, and Turkey (Basurco 2000; Bravo and Montañes 2001). The situation is quite different for most fish farms that have been installed in some western Mediterranean countries in the last decade. Progressive advances in cage building now facilitate mooring of cage farms on relatively deep bottoms and exposed sites. Indeed, about 50% of the 45 production units operating in Spain in 1998 had cages in semi-offshore and offshore conditions (Basurco and Larrazabal 2000).
Fish farms in semi-exposed conditions are proliferating in the western Mediterranean on the assumption that enhanced water renewal in cages provides improved culturing conditions. Increased fish production is expected to compensate for greater monetary investments and higher damage risks. From an ecological point of view, it is assumed that enhanced water renewal in semi-exposed conditions may result in less environmental impact than is found in farms in semi-enclosed bays. If so, the environmental problems described in the literature for more traditional cage farms may not reflect the traits of the impact exerted by Mediterranean fish farms at semi-exposed sites appropriately. To contribute to the clarification of this issue, we have selected five semi-exposed cage farms and have assessed their environmental impact by examining diverse standard water-column and benthic parameters.
Methods
Farm features
We studied five semi-exposed cage farms scattered over 900 km along the Mediterranean coast of Spain (Fig. 1), spanning a wide range of hydrographic and environmental conditions. All farms grow sea bream (Sparus aurata) and/or sea bass (Dicentrarchus labrax), but average fish biomass per farm during 2002 ranged from to 40 to 549 tons, depending on farm (Table 1). Therefore, the study considers a variety of conditions, not only environmental but also farming. Most farms were less than 6 years old, so that they can be considered modern installations. They all use either 19 or 25 m diameter floating cages moored on soft bottoms between 21 and 37 m deep (Table 1). Fish feeding is monitored by underwater video cameras, allowing optimal adjustment between fodder supply and uptake by fish.
General sampling procedures
To investigate the potential environmental effects of fish farming, we examined a set of parameters relative to the water column (i.e., concentrations of four inorganic dissolved nutrients and heterotrophic bacteria) and a set of parameters relative to the bottom (i.e., concentration of benthic pigments and organic matter in sediment, size-grain structure of sediment, and abundance of benthic macroinvertebrates). We evaluated the impact level by comparing parameter values between samples from fish farms and samples from control zones. Each farm had a control zone located about 3 nautical miles away from the farm and in the opposite direction to the prevailing current in the area in order to minimize potential interactions with dispersed farm wastes. We selected control zones on a bottom as similar as possible to that of the corresponding fish farm, also characterized by similar depth (±3 m) and distance from shore (±80 m).
Samples were collected bi-monthly in March, May, and July 2003, capturing the transition between pre-summer and summer environmental conditions, which is likely the most dynamic and complex environmental situation in the Mediterranean coastal system. Here, we assumed that sampling all year round is not necessary, because if crucial environmental and biotic parameters are affected by fish farming, differences between controls and farms should be detectable at any time. Sampling was conducted by divers early in the morning before fish feeding. Samples were immediately sent by refrigerated courier to the Centro de Estudios Avanzados de Blanes (CEAB-CSIC), where we carried out the analyses within 36 h of sampling.
Dissolved nutrients and bacterioplankton
We investigated total concentrations of four inorganic dissolved nutrients: orthosilicic acid (hereafter referred to as “silicate”), nitrate, nitrite, and phosphate. Although silicate does not occur in farm wastes, we investigated silicate levels around the farms to evaluate the possibility that inputs of nitrate and phosphate from farms in areas with elevated dissolved silicate concentrations of natural origin could fuel diatom blooms. In this approach we failed to measure nitrogen compounds derived from fish excretion directly, which consists of up to 85–90% ammonia (NH +4 ) and 5–10% urea (Dosdat 2001). After excretion, ammonia is quickly oxidized to nitrate, and urea transformed in ammonium, which is rapidly taken by both phytoplankton and bacteria. Therefore, although we did not measure nitrogen compounds from fish excretion, we evaluated nitrate values and the potential effects of excess ammonium in bacterioplankton and benthic chlorophyll. We discarded measurements of planktonic chlorophyll because this parameter has been shown to be a bad descriptor of fish-farm impact due to rapid water replacement around the cages (Pitta et al. 1999), a circumstance that particularly applies to our study of semi-exposed farms.
By using 250-ml polypropylene bottles, we sampled seawater (n=5) for nutrient determination in fish farms 1 m below the ocean surface (surface water) and 1 m above the seafloor (bottom water). We measured concentration of nutrients immediately upon the arrival of samples at the laboratory using a TRAACS-2000 Autoanalyzer.
We investigated concentrations of heterotrophic bacteria by subsampling 3 ml seawater from the polypropylene bottles. Subsamples of surface and bottom seawater from each fish farm and control (n=5) were delivered to 1.5 ml criovials, fixed in 10% paraformaldehyde (1%) plus glutaraldehyde (0.05%) at room temperature in the dark for 10 min (Marie et al. 1996). Samples were then stored at −80°C until bacterial counting, which took placed within 2 weeks. We used a FACScalibur flow cytometer emitting at 488 nm, in which bacteria are detected by their signature in a side scatter versus green fluorescence plot, after defrosting and fluorescent staining with 1.6–5 μM Syto 13 (Molecular Probes) for 15 min in the dark (Del Giorgio et al. 1996; Gasol and Del Giorgio 2000). Count calibration was provided by adding 5% of a 106 ml−1 solution of yellow-green 0.92 Polyscience latex beads to water samples. Counts were based on sample runs at low speed (approximately 60 μl min−1) and data stored in a log model for 2 min or until 1,000 events had been acquired. All samples were processed undiluted.
For each fish farm, we examined differences in concentrations of nutrients and bacteria as a function of “zone” factor (farm zone versus control zone) and “depth” factor (surface water versus bottom water) using two-way ANOVA. When data did not meet the assumptions for the ANOVA, we applied appropriate transformations, as indicated in the graphs.
Given that sampling size is moderate (n=5), we avoided three-factor designs, discarding less relevant factors that would complicate the statistical approach and weaken the power to detect effects of more relevant factors. For instance, examination of between-farm differences in nutrients and bacteria would be of little interest in terms of impact evaluation, because putative between-farm differences likely reflect geographical distance between farms. Similarly, examination of differences between sampling times would be of minimum interest in terms of impact detection, because nutrients and bacterioplankton are known to show marked seasonal changes in the Mediterranean. Consequently, we obviated between-farm variability, also statistically analyzing samples from March, May, and July separately. Nevertheless, for instructive purposes, the results are presented in graphs, the format of which allows easy inspection of between-farm and between-time variability.
When significant differences in nutrient or bacteria concentration were detected by the two-way ANOVA, we run Student–Newman–Keuls (SNK) “a posteriori” tests to identify the groups responsible for the differences. Because we are particularly interested in examining differences between farm zones and their respective controls, and because SNK tests based on small sample size (n=5) may have limited power to detect significant differences, we specifically re-analyzed differences between each individual farm and control by time and water type (surface water versus bottom water) using either the parametric t-test or the non-parametric Mann–Whitney rank sum test. The results of both pairwise analyses were consulted before concluding that significant differences between farm and control sites had occurred.
Pigments and organic matter in sediment
We investigated the content of pigments and organic matter in the 0.5 cm-thick upper layer of sediment, which was collected by divers drawing 50 ml polystyrene Falcon tubes at the seabed.
For pigment analyses, 3-g sediment sub-samples (wet weight) were extracted in 90% acetone. Extraction consisted of three steps, intercalated with 5-min centrifugations at 2,000 rpm. After each centrifugation, the supernatant was collected using a syringe and subsequently filtered through a 2 μm-pore filter. All three supernatants of each sample were mixed and centrifuged at 3,500 rpm for 5 min to minimize turbidity prior to spectrophotometer reads. Sample absorbance was measured using a SHIMADZU UV-2100 Spectrophotometer at 750 and 665 nm before and after acidification with 0.1 N of HCl, respectively. By using Lorenzen’s equations with the appropriate corrections for sediments samples (Lorenzen 1966), absorbances were respectively transformed into concentration of chlorophyll a, which provides information about the abundance of benthic microalgae, and phaeophytin. The latter is a breakdown product of chlorophyll, and the ratio of chlorophyll to phaeophytin is used to indicate the health of the microalgal assemblage.
For the analysis of organic matter, we used 3-g (dry weight) sediment sub-samples. After homogenization and drying at 60°C to constant weight, sediment was combusted in a Muffle furnace at 500°C for 5 h. The amount of organic matter in a sample was estimated as the difference between dry and ash weight.
We examined differences in chlorophyll a, phaeophytin, and organic matter as a function of zone (farm zone versus control zone) and farm factor (farm no. 1 – no. 5) using a two-way ANOVA (n=5). When data did not meet the assumptions for the ANOVA, we applied appropriate transformations, as indicated in the graphic results. When significant differences were detected by the ANOVA, we ran SNK tests to identify the groups responsible for the differences, paying special attention to pairwise comparisons between each farm and its corresponding control. Samples from March, May, and July were analyzed separately to avoid a low-power three-factor approach.
Granulometric structure of sediment
We investigated the grain-size structure of sediment using 100 g (dry weight) samples collected by a diver-operated hand corer. Formaldehyde-fixed samples were oxidized in 6% hydrogen peroxide for 48 h and dried at 60°C to constant weight. Subsequently, we processed sediment using an electrical CISA sieve, which separates sediment into eight grain-size groups (i.e., x<0.06 mm, 0.06 mm<x<0.12 mm, 0.12 mm<x<0.25 mm, 0.25 mm<x<0.35 mm, 0.35 mm<x<0.5 mm, 0.5 mm<x<0.75 mm, 0.75 mm<x<1 mm, x≥1 mm).
Because dredging and the addition of foreign sand for beach restoration is a common activity at some of the studied localities from March to June, we determined sediment structure in March (when a natural post-winter situation is expected) and July (when an artificial pre-summer situation is expected). Granulometric structure was expressed as weight percentage of the various grain-size fractions, and fractions named according to the Udden–Wentworth scale (Wentworth 1972).
Additionally, we estimated quantitatively the similarity among the soft bottom of the diverse farms and control zones using classification analysis. The analysis was based on the untransformed average (March and July samples) of weight percentage for each of the eight grain-size fractions plus chlorophyll a. We calculated pairwise granulometric distances between all 10 zones using the Bray-Curtis distance (BCd), which is semimetric and does not consider the shared absence of descriptors (Legendre and Legendre 1998). For a more intuitive interpretation, BCd can easily be converted into its complement, the semimetric Steinhaus similarity (Ss=1−BCd). Finally, the BCd matrix was processed by the UPGMA clustering algorithm to produce a cladogram of zones based on “sediment distances”.
Macroinvertebrate fauna
For the faunal analysis, we collected one hand-corer (10 cm×15 cm) by Scuba diving at each zone in March, May, and July. Sediment samples were fixed in 4% formaldehyde and sieved through a 500 μm-mesh sieve to separate the macroinvertebrates. The organisms were preserved in 70% ethanol, stained with 1% Bengal Rose, then counted and identified under dissecting and compound microscopes. Animals were classified to the family level, an approach that provides an efficient “research effort/result resolution” ratio when assessing the environmental impact of fish farming (Karakassis and Hatziyanni 2000).
To increase test power in detecting faunal differences between control and farm samples, we pooled samples collected from March, May, and July, disregarding the examination of differences as a function of “time” factor. Then, we investigated faunal differences between controls and farms at three levels of complexity. First, we examined differences in taxonomic richness and diversity between farm and control zones. Subsequently, we used cluster analysis to explore faunal differences between a given farm and its control in comparison to those between each of the remaining farms and their respective controls. Finally, we investigated the level at which the environmental variables under study (i.e., dissolved nutrients, bacterial concentration, grain-size structure of sediment, benthic chlorophyll a, and organic matter in sediment) are responsible for differences in faunal distribution between control and farm zones using both unconstrained and canonical ordination analyses.
Taxa richness was estimated as number of families represented in each zone. Biodiversity was estimated by the unbiased Simpson’s Diversity Index (D), as proposed by Pielou (1969). The value of D ranges between 0 and 1; the greater the values, the greater the sample diversity. To examine differences in D values between farm and control zones, we used a paired t-test, after checking data for normality and homoscedasticity. We used a paired test rather than a regular (non-paired) t-test, because diversity values for each farm and its control are non-independent statistically, i.e., are associated because of geographical closeness.
At a second level of analysis, we examined quantitative faunal differences between a given farm and its respective control by calculating pairwise BCd. Subsequently, the BCd matrix was processed by the UPGMA clustering algorithm to produce a cladogram of faunal distance among zones. In this analysis, we considered all taxa collected in the study and analyzed their abundances as untransformed data.
Finally, we assessed the explainable faunal variation in the “zone per taxa” matrix using unconstrained correspondence analysis (CA). Then, we used canonical correspondence analysis (CCA) to estimate the portion of variation related to the environmental variables measured in this study. Because rare taxa may produce distortion of ordination scores, we excluded taxa with abundance values <3 from these analyses. In addition, we down-weighted rare species, using the option available in the CANOCO 4.0 software. Given that impacted communities are often characterized by dramatically uneven distributions of abundance values across taxa and sites, we ran the analyses on untransformed abundances rather than on log-transformed ones to maximize the chances of detecting anomalies in abundance distribution.
In CCA analyses, we initially considered a total of seven variables; three of them related to the benthic component (i.e., average percentage of chlorophyll a, organic matter in sediment, and mud in sediment), the four others to the water column (i.e., average concentration of phosphate, nitrite, nitrate, and bacteria in bottom water). Concentration of silica was not considered in these analyses because it is not really fish-farm waste. The relevance of the environmental variables to be considered in the analyses was checked by preliminary “manual addition” tests, as well as by inspection of the variable inflation factors (VIF) of trial models. These exploratory CCAs revealed that, although the global model based on these seven variables is statistically significant, “bacteria” and “organic matter” are redundant variables, explaining a portion of variation also explained by the “mud” variable. Therefore, we ran a second five-variable analysis considering just three water-column variables (i.e., phosphate, nitrate, nitrite) and two benthic variables (chlorophyll a and mud).
In the five-variable approach, we examined the environmental sources of variation within the “taxa per zones” matrix, discriminating the variation uniquely described by each set of variables (i.e., water-column versus benthic ones), that jointly explained by both sets, and that unexplained by the analysis (Borcard et al. 1992). The statistical significance of the first canonical axis and all canonical axes of the resulting models were tested by the Monte-Carlo test using 500 permutations under the reduced model or under the full model if co-variables were considered in the analysis.
To generate bi-dimensional ordination diagrams, in which zones and taxa are represented by points and quantitative environmental variables by vectors (in CCA), we opted for a biplot scaling focused on inter-zone distances.
Results
Dissolved nutrients and bacterioplankton
Silicate
The most obvious global trend is that the concentration of silicate progressively decreases from spring to summer in both control and fish-farm zones, also in both surface and bottom waters (Fig. 2a). This is likely the result of the spring bloom characterizing the populations of western-Mediterranean diatoms, which are major silicate consumers. Despite major global patterns, a more detailed analysis of silicate concentration for each fish farm indicates a disparity of trends as a function of zone and depth factors, depending on fish farm. It is noteworthy that zone factor was statistically significant in only five out of the 15 analyses, while depth factor was significant in nine analyses. More importantly, in those cases in which zone factor was significant, the SNK tests indicate that the sign of the difference between farm water and control water is not consistent; sometimes concentration is higher in farms and sometimes in controls, depending on farm, depth and time.
Concentration (mean ± SD) of silicate (a), phosphate (b), nitrite (c), and nitrate (d) in water samples collected from surface and bottom water of control (C) and farm (F) zones in March, May, and July 2003. Bold uppercase letters at the upper left corner of graphs refer to main factors (Z zone, D depth) and their interaction (Z×D) in the 2-way ANOVA. Statistically significant factors are underlined. A–D letters at the upper right corner refer to mean values arranged in decreasing magnitude. Groups of underlined letters indicate non-significant differences between pairs of means according to an “a posteriori” SNK test (P<0.05) following the ANOVA. When data transformations were applied prior to analysis, it is indicated by a single letter placed between the results of the ANOVA and the SNK tests, as follows: L log 10 transformation, R rank transformation, S square transformation
Phosphate
Phosphate concentration remained consistently low over the study period, in both control and fish-farm areas, also in surface and bottom waters (Fig. 2b). This is consistent with the phosphorous-limited nature of the western Mediterranean (Hargrave et al. 1991). We also noticed that the sign of differences in phosphate concentration as a function of zone and depth shifted depending on fish farm and sampling time. In all cases, differences were very small in magnitude. Although the ANOVAs do not clearly support a consistent zone effect on phosphate concentration, it is noteworthy that zone factor was significant in eight out of the 15 analyses, with farm bottom water ranked as either category A or B in 11 out of the 15 groups of SNK tests. This suggests a subtle trend in average phosphate concentration of farm bottom water to be slightly increased relative to the remaining water types. Therefore, we cannot discount small inputs of phosphate into the bottom seawater under the cages.
Nitrite
There is a progressive decrease in nitrite concentration in both control and farm zones of most fish farms from spring to summer, affecting both surface and bottom waters (Fig. 2c). However, detailed analyses for each fish farm indicate that zone and depth factors are also additional sources of variation in nitrite distribution, depending on fish farm and sampling time. The fact that zone factor was statistically significant in only three out of 15 analyses supports the theory that fish farming activities do not systematically affect nitrite concentration around farms on a local scale. Nevertheless, should any potential risk of nitrite contamination be assessed in further detail, most research effort should be focused around March. At this time, we detected a trend in nitrite values to be increased relative to controls in four out of the five studied farms, a situation that reverses in May and July.
Nitrate
Nitrate concentration progressively decreases from spring to summer in both control and farm zones, also in surface and bottom waters (Fig. 2d). Like in the above nutrients, a detailed analysis for each fish farm indicates that zone and depth factors are additional sources of variability in nitrate distribution, depending on fish farm and sampling time. Zone factor was significant in eight out of 15 analyses, with SNK tests suggesting that in most cases nitrate concentration under the cages is lower than in controls. Such a pattern goes against the general prediction that remineralization of fodder should produce increased concentrations of nutrients under the cages.
Bacterioplankton
Unlike dissolved nutrients, the average concentration of heterotrophic bacteria increases slightly from March to July, particularly in bottom water (Fig. 3). Fish farm no. 3 is an exception to this pattern. It is also noteworthy that bacterial concentration increases slightly from fish farm no. 1 to no. 5, i.e., from south to north along the Spanish Mediterranean coast. Again, farm no. 3, which is characterized by comparatively high concentrations of bacteria in both control and farm zones, does not match this trend.
Bacterial concentration (mean ± SD; expressed as 106 cells ml−1) as a function of zone factor (farm zone versus control zone) and depth factor (surface water versus bottom water) in March, May, and July samples. For a description of the letters, see Fig. 2
Detailed analysis revealed that zone and depth are additional sources of variation in bacterial concentration. However, the sign of variation depended on fish farms and sampling time, with no consistent pattern. Zone factor was significant in nine out of 15 analyses, but the sign of differences in bacterial concentration between farm and control zones was not consistent across fish farms or sampling time. Therefore, it seems that fish farming does not play a relevant role in modifying natural values of bacterial concentration in the seawater surrounding the farms, except for around farm no. 3. Depth factor appears to be more relevant than zone factor in determining bacterial concentration, since bottom water was usually richer in bacteria than surface water, irrespective of zone (Fig. 3). This pattern appears to be particularly marked in July.
Pigments and organic matter in sediment
The average chlorophyll a content was relatively low, ranging from nearly undetectable levels to about 1.6 μg g−1 dry sediment (Fig. 4). A significant farm factor in March, May, and July indicated marked differences between farming installations. In March and July, zone factor was also significant. However, SNK tests and additional pairwise t- or U-tests revealed that, in at least some cases, the concentration of benthic chlorophyll under the cages was lower than in controls. This appears to be the general pattern in May, though lacking statistical support.
Concentration (mean ± SD) of chlorophyll a and phaeopigments in sediment samples collected in March, May, and July 2003. Bold uppercase letter at the upper right corner of graphs refer to the main factors (F farm factor: farm no. 1 – no. 5; Z zone factor: control zone versus farm zone) and their interaction (F×Z) in the 2-way ANOVA. Statistically significant (P<0.05) factors are underlined. Asterisks indicate significant differences (P<0.05) in pigment content between each farm zone and its corresponding control zone, according to the SNK tests following the ANOVA; non-significant differences are indicated by ns. Disagreement between SNK tests and additional t-tests or non-parametric Mann–Whitney (U) for differences between each farm zone and its corresponding control zone is indicated by a question mark
The analysis of phaeophytin revealed that all sediment samples other than those from fish farm no. 4 contain relatively important amounts of chlorophyll degradation products (Fig. 4). The ANOVA detected marked differences between farming installations at all three times, also significant differences between farm zone and control zone in May and July. Like for chlorophyll, there is a trend in phaeophytin content to be higher in control than in farm sediment, except for fish farm no. 3 in July. However, such a trend was only statistically confirmed by SNK tests and t or U tests in a few cases (Fig. 4). Unattached calcareous macroalgae, occasionally brought to both farm and control zones of fish farm no. 3 by storms from a close, deeper pre-coralligenous community, are responsible for the very distinctive values of chlorophyll and phaeopigments in this farm.
Levels of organic matter in sediment samples were relatively low, averaging less than 1% in the mobile coarse-sand bottoms of farm no. 4 and its control (Fig. 5). There were significant between-farm differences in March, May, and July, but significant between-zone differences occurred in March and May only. SNK tests and additional pairwise tests for comparison between farms and controls supported no clear pattern. For instance, while organic matter in control sediment was significantly higher than in farm sediment in fish farm no. 1, the opposite trend was observed in fish farm no. 3, particularly in July.
Content (mean ± SD) of organic matter, expressed as dry weight percentage, in sediment samples collected in March, May, and July 2003. Letters and symbols are the same as in Fig. 4
Granulometric structure of sediment
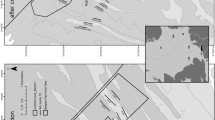
Remarkable similarity was found in grain-size structure between the sediment of control and farm zones in fish farms nos. 2, 4, and 5 in March, while there were clear differences in farms nos. 1 and 3 (Fig. 6). Samples of control no. 1 mostly consisted of mud, while those from farm zone no. 1 were mostly coarse and medium sand. At farm no. 3, the pattern is the opposite, with the control zone mostly consisting of coarse and medium sand and the farm zone of fine and very fine sand. In addition, we found that the granulometric structure of sediment is not a permanent feature around some farms. Substantial granulometric variation occurred between March and July in farms nos. 2 and 4, mostly due to an important increase in coarse sand, likely the result of both severe storms and dredging for beach restoration during spring. The input of coarse sand increased the similarity in granulometric structure between control and farm zone no. 4, and decreased the between-zone similarity in farm no. 2.
The Steinhaus index corroborated that control and farm zones were similar in sediment structure for farm no. 2 (62%), farm no. 4 (79%), and farm no. 5 (92%). In contrast, between-zone similarity was low for fish farm nos. 1 (25%) and 3 (19%). A dendrogram based on BCd reveals that farm zone no. 1 was similar to both zones (control and farm) of fish farm no. 4 (Fig. 7), all three being characterized by high percentages of coarse and medium sand (Fig. 6). In contrast, the control zone of fish farm no. 1, characterized by a high percentage of mud, is unlike any other zone in this study. The situation is different for fish farm no. 3. Control zone no. 3 goes with the zones of farm no. 4, characterized by coarse and medium sand, while farm zone no. 3 clusters with the zones of farm no. 2, characterized by a high proportion of fine sand. Hence, granulometric differences in sediment structure between control and farm zones in fish farms nos. 2 and 3 may be potentially responsible for some differences in faunal composition, making the detection of faunal differences due to farming activity difficult.
Macroinvertebrate fauna
A total of 7,042 organisms belonging to 104 families and 10 phyla were collected (Fig. 8). In addition, one ophiuroid, two bivalves, and five amphipods of open family affiliation were regarded as “incertae sedis” within their respective phyla. Polychaetes (40 families), amphipods (16 families), and bivalves (14 families) are the most representative groups. It appears that the number of families occurring in farm zones is lower than that found in their respective control zones, except for farm no. 1 (Fig. 9). On average, control zones contained 41±9.62 families, whereas farm zones contained 31±12.66 families. This trend suggests biodiversity reduction in farms relative to controls and agrees with the results of the Simpson’s diversity index, which reaches consistently higher values in controls than in farms (Fig. 9). On average, diversity was 0.92±0.01 in controls, but 0.60±0.32 in farms. However, such differences in biodiversity between farms and controls are not statistically significant when analyzed by a paired t-test (n=5, t=−2.22, P=0.090), an effect probably due to insufficient power in the analyses (see Discussion).
The Ss index and a cladogram (Fig. 10) based on faunal Bray-Curtis distance (BCd) indicated that faunal similarity (Ss×100) between farm and control zones was moderate to high for fish farms nos. 2 (68.5%) and 4 (40.6%), but lower for fish farm no. 5 (22.5%). Faunal similarity between control and farm zones was extremely low for fish farms nos. 1 (10%) and 3 (6.7%). Because a cladogram based on sediment features showed that there are important differences in sediment structure between control and farm zones in these two fish farms (Fig. 7), it remains unclear how much faunal difference is due to sediment differences and how much is due to fish farming (see Discussion).
The first four axes extracted in the correspondence analysis (CA) explained 75.4% of faunal variation in the “taxa per zones” matrix (Table 2). Similar variation (i.e., 73.5% and 72.4%) was explained by a canonical correspondence analysis (CCA) based on seven and five environmental variables, respectively. In addition, these two CCAs explained 80.5% and 93.6% of variation in the “taxa-environment” matrix, respectively (Table 2). In both CCA analyses, Monte-Carlo Permutation tests indicated that both the first axis (P=0.01) and all canonical axes together (P=0.002) were highly significant, indicating that the environmental variables under study are clearly responsible for at least some of the variation within the “taxa×zones” matrix. We present here only the results of the five-variable model, the VIF of which ranged from 1.1 to 2.1, indicating that “phosphate”, “nitrate”, “nitrite”, “chlorophyll a”, and “mud” are relevant, non-redundant variables. This was not the case for the seven-variable model, the VIF of which ranged from 8 to 140. The variance partitioning analysis (Table 3) indicates that the set of water-column variables (54.11% of variation) is about 2.5 times more important than the benthic set (22.14%) in explaining faunal variation. More importantly, neither set of variables is redundant, because shared explained variation is only 1.2%.
The bidimensional ordination space of the unconstrained CA showed that, from a faunal point of view, farm and control zones do not appear clearly separated except for fish farms nos. 1 and 3 (Fig. 11). The CCA corroborated the faunal trend revealed by the CA, depicting farm zones nos. 1 and 3 as markedly different not only from the remaining zones, but also from each other (Fig. 12a). The fact that axis 1 of both ordination analyses does not clearly discriminate farm from control zones suggests that fish farming activity does not cause the macroinvertebrate community under the culturing cages of the different farms to converge towards a typically impacted community with high abundance of a few opportunistic taxa. Rather, the first ordination axis appears to be associated with variation in benthic variables, while the second axis appear to be related to variations in water-column variables (Fig. 12a). In the CCA biplot, only the position of farm no. 4 has changed in an appreciable way relative to the environmentally unconstrained CA. This may indicate that the influence of environmental variables on the distribution of faunal abundances is just moderate. Indeed, the shift of position for farm zone no. 4 towards the coordinate center indicates that it is characterized by taxa with abundance values little related to the ordination axes constrained to the environmental variables. In contrast, faunal abundance in farms nos. 1 and 3 appears to be associated with some atypical conditions, such as high phosphate concentration in the bottom water of farm zone no. 1 and a bottom rich in mud, bacteria, and organic matter in farm zone no. 3.
a Biplot showing the bidimensional space formed by the first (X) and the second (Y) axes of a 5-variable CCA, depicting the relative positions of the 10 studied zones (C control zone, F farm zone) in relation to the environmental variables (solid vectors). Dotted vectors indicate the approximate position of “organic matter” and “bacteria” variables, when considered in a 7-variable CCA model. b Bidimensional space formed by the first (X) and the second (Y) axes of a CCA in which the relative positions of the taxa responsible for the location of farm zones nos. 1, 3, and 4 are shown
The “taxa” scores reveal that the distinctive character of the fauna under the cages of farm no. 1 derives from a high abundance of errant carnivorous polychaetes of the family Dorvilleidae and exclusive low abundance of some bivalves (lucinids and corbulids) and leucothoid amphipods. Neither of these taxa are known to be particularly associated with conditions of increased organic matter or polluted habitats. In contrast, the peripheral location of farm zone no. 3 in the ordinations is favored by a high abundance of capitellid polychaetes (virtually all in the Capitella capitata species complex) and nassarid gastropods, both of which are well-known bioindicators of organic pollution in benthic coastal systems.
Discussion
Dissolved nutrients and bacterioplankton
There is the general prediction that continued inputs of dissolved nutrients from farming activities usually result in drastic environmental changes that alter both planktonic and benthic communities at the local scale. In our study, we found that neither surface nor bottom waters at the fish farms showed abnormal concentrations of nitrite and nitrate relative to controls. Nitrates, which are important source of nitrogen for phytoplankton growth, tend to be decreased under the cages. This is unlikely to be the result of uptake by phytoplankton, as chlorophyll a values under the fish cages were low relative to control sites. Belias et al. (2003) have attributed the nitrate decrease in the vicinity of fish farms to anaerobic consumption. Nevertheless, this does not appear to be the case in the studied farms, in which neither the structure of sediment nor the biotic parameters indicated anaerobic conditions under the cages.
We detected a generalized decrease in nutrient concentration from spring to summer. This reflects the typical dynamics known for the western Mediterranean, caused by summer stratification of the water column due to shallow pycnoclines and maximum phytoplankton growth and nutrient uptake in the upper water layer due to increased temperature and irradiance. We also found incomplete evidence suggesting small inputs of inorganic phosphorous into farm bottom water, particularly under summer conditions. Such an issue may be worth further investigation, given that the Mediterranean is a phosphorous-limited sea (Krom et al. 1991) and even minimum inputs can have potentially drastic effects on the planktonic system. Our results confirmed that variability in silicate concentration is not related to farming activity, but dependent on natural processes (e.g., expansion of diatom populations, seasonal formation or disruption of thermoclines, etc.). Therefore, the possibility of diatom blooms favored by excess nutrients in the surroundings of the studied fish farms is negligible.
So far, relatively few studies have addressed the issue of dissolved nutrients in Mediterranean fish farms and no general pattern has emerged. Ruiz et al. (2001), who investigated dissolved nutrients in a large (700–800 tons fish per year) fish farm located in an embayment at the Murcian coast (Spain, Mediterranean coast), reported that major differences in nitrate and nitrite concentrations are explained by seasonal changes in the environment rather than by fish-farming activities, a pattern consistent with our results. Concentrations of nitrate and nitrite were significantly higher in winter than in summer, a situation similar to that found in our study. Likewise, they also found that the concentration of phosphate in the farming area was higher than that in controls (up to three-fold). Pitta et al. (1999), who investigated dissolved nutrients and chlorophyll a in three medium-sized Greek fish farms located in semi-enclosed bays or sheltered locations, found no significant differences in dissolved nutrients and chlorophyll a between farms and control sites in two out of three studied farms. Consistent with our results, they reported that phosphate in farm water was increased and nitrate decreased relative to control water for a third fish farm located in an embayment at Ithaki Island. While we detected just subtle differences between farm and control zones in our semi-exposed fish farms, Pitta et al. (1999) reported marked differences, with phosphate in the cages being up to 5.3-fold higher than at the control site, and nitrate being up to 12.5-fold lower than in the control. This is consistent with the idea that semi-exposed cage farms favor rapid dissipation of the effects of dissolved nutrients in the effluent. This hypothesis is also supported from the results of Wu et al. (1994), who detected an increase in dissolved nutrients only in farms with poor tidal flushing and high density of fish in cages. Unfortunately, we failed to measure ammonium concentrations in our water samples. Several studies have pointed out that ammonium may be an abundant form of inorganic nitrogen in the effluent of fish farms (Hall et al. 1992; Wu 1995; Karakassis et al. 2001; Pitta et al. 1999; Ruiz et al. 2001). A study of sea-bass excretion by Dosdat (2001) revealed that a large percentage of the ingested nitrogen is excreted in a dissolved form as ammonia (85–90%) and urea (10%), which readily transform into nitrate and ammonia, respectively, thus becoming available for the phytoplankton and part of the bacterioplankton. Nevertheless, our results suggest that the impact of fish excretion appears to be of little relevance in the studied farms as we found that neither the bacterioplankton nor the benthic microalgae are increased in the vicinity of farms. Similarly, Pitta et al. (1999) found that increased ammonium concentration in a fish farm had no significant effect on chlorophyll concentration in the water column. Indeed, regarding the heterotrophic bacterioplankton (cyanobacteria not considered), we found that bacterial concentration varies greatly between farms, between spring and summer, and between surface and bottom water, but very rarely does it vary between farm and control zones as the result of fish-farming activity. Bacterial concentration in the vicinity of fish farms has been investigated in very few studies. Consistent with our results, Alongi et al. (2003) found differences in concentrations of bacteria as a function of location, depth, or tides, but no differences between farms and control sites. In contrast, Mirto et al. (2000), who measured bacterial abundance in sediment, not in water samples, found higher concentrations of bacteria under a mussel farm than in the control area. Similarly, Vezzulli et al. (2002) found that the bacterial content of sediments under fish cages was up to three times higher than that of control station. Therefore, the little information that is available suggests that populations of planktonic bacteria react differently to wastes from fish farming than benthic bacteria, which appear to be better environmental indicators.
Pigments and organic matter
Many studies have pointed out that the input of organic matter rich in phosphorous and nitrogen into the sediments under the cages is the most obvious source of environmental impact by cage farms (e.g. Hargrave et al. 1993, 1997; Fabiano et al. 1995; Delgado et al. 1997, 1999). Fish-farming organic matter is assumed to remineralize faster than “natural” organic matter, which consists mostly of refractory plant material and has a very high C:N ratio. Rates of organic matter accumulation on the seabed under the cages are known to vary from farm to farm, mostly influenced by local hydrological and geomorphologic features, also depending on fish production and fodder quality. As a result of these differences, accumulation rates reported in the literature range from nearly irrelevant to important. For instance, in a large (700–800 fish tons per year) farm located over a 25-m deep bottom at an embayment on the coast of Murcia (Spain, western Mediterranean), Ruiz et al. (2001) found mean values of organic matter (ash weight %) in sediments ranging from 0.5% to 0.8% under the cages, and from 1.9% to 2.4% in a reference area. In contrast, in a slightly larger farm (1,000 tons fish per year) located over a 20-m deep bottom in Cephalonia bay (Greece, eastern Mediterranean), Karakassis et al. (1998) reported that organic matter contribution to the upper most sediment layer ranged from 20 to 40% under the cages and around 10% at the control site. We have found relatively low organic matter levels. They were exceptionally low (<1%) both below the cages of fish farm no. 4 and at its control site, the bottoms of which are 37 m deep and consist of highly mobile coarse sand. There were no significant differences in the content of organic matter between farms and their respective controls, except for farm no. 3. Because all five fish farms studied are at relatively deep, semi-exposed sites, coastal currents and distance to the bottom are assumed to facilitate dissipation of the effects of suspended solid and dissolved nutrients in the effluent. The organic matter generated is likely to be found scattered over a wide “action zone” surrounding the farms, rather than directly under the cages. In addition, the fact that the studied fish farms monitor consumption of fodder by fish using underwater cameras helps to prevent excess uneaten fodder at the bottom. More importantly, we postulate that the large aggregations of wild fish detected around floating cages (Dempster et al. 2002) are likely to consume most of the fodder that escapes from the cages, minimizing the organic enrichment of the seafloor. Field experiments have revealed that about 80% of particulate organic matter escaping from the cages may be consumed by wild fish before reaching the seafloor (Vita et al. 2004). Therefore, determination of the fate of the particulate organic wastes generated by semi-exposed cage farms may not be easily predictable and may require empirical determination for each farm in association with both a biochemical characterization of the sediment and a hydrodynamic characterization of the farm surroundings. This view is consistent with the results of recent studies on other sea-bream and sea-bass farms, that suggest that organic carbon concentration itself does not adequately describe the ecological impact of fish farming relative to a control site (Karakassis et al. 1998; Mazzola et al. 2000; Molina et al. 2001; Vezzulli et al. 2002). Nevertheless, detailed analysis of organic matter composition by Mazzola et al. (1999) revealed comparable high concentrations of proteins and carbohydrates between the farm and the control site, but a distinctive higher lipid content in farm sediments than in control sediments. These authors propose the use of lipid biomarkers as a tool to determine in more detail the spatial scope of fish-farming impact on coastal sediments.
Regarding the chlorophyll a content in sediments, we found mean values ranging from 0.2 to 0.8 μg Chl a per g of sediment, depending on fish farm. These values are unusually low. At first sight, it appears that benthic chlorophyll tends to be lower in farm than in control zones, although such a trend gets poor statistical support (Fig. 4). This chlorophyll distribution pattern conflicts with those reported from several other Mediterranean farms. Mazzola et al. (1999, 2000) found pigment concentrations in farm sediment two times higher than that in control sediment. A similar pattern has been described from other Greek farms at Cephalonia Bay (Karakassis et al. 1998) and from a small (16 tons fish per year) farm located above a 10-m deep bottom at La Spezia Gulf (Ligurian Sea, Italy; Vezzulli et al. 2002). We postulate that in the studied farms there is a coincidence of circumstances responsible for a depauperate phytobenthic community: the shading effect of floating cages; no significant nutrient enrichment in the vicinity of cages; and relatively deep bottoms. Nevertheless, the atypically low chlorophyll values that we are reporting may not adequately represent the real in situ values, being probably affected by some chlorophyll degradation during the transportation of samples in refrigerated (4°C) rather than frozen conditions. The analysis of differences in phaeophytin content as a function of zone (farm zone versus control zone) revealed that phaeophytin in control sediment tended to be higher than in farm sediment. This indicates that the phaeophytin content in our samples probably does not reflect phytobenthos–phytoplankton degradation in the vicinity of farms, but rather chlorophyll degradation during sample transportation. This suspicion was corroborated when we found a strong significant correlation (not shown) between sample phaeophytin content and geographical distance between the sampling location and the laboratory; phaeophytin was hardly detectable in samples collected at fish farm no. 4 (Fig. 4), which is at the same location as our laboratory. Therefore, our conclusion that chlorophyll a content is significantly lower in farm sediments than in control sediments is very reliable, since “real” differences in the magnitude of chlorophyll concentration between farm and control zones are actually larger than those we submitted to statistical analysis. On the other hand, although our estimates of chlorophyll content are valid for comparisons between farms and controls, they are unreliable for either between-farm comparisons or comparison with other values available in the literature.
Macroinvertebrate fauna
Preliminary inspection of taxa richness and biodiversity suggests that both parameters show lower values in farm zones than in controls, but such a trend gets poor statistical support from our analyses (Fig. 9). The power of our paired t-tests, which was just 32%, may be partially responsible for a non-significant effect: better statistical resolution would require consideration of a higher number of farms in the study. Nevertheless, the faunal analyses based on clustering and ordination techniques were partially consistent with the results of the biodiversity analyses, indicating no major faunal difference between farm and control zones in fish farms nos. 2, 4, and 5. Therefore, fish-farming appears to have a weak impact on the macroinvertebrate fauna at these locations (Figs. 10, 11).
Major differences in composition and abundance distribution of fauna between farm and control zones were detected for fish farms nos. 1 and 3 only. Based on sediment similarities (Fig. 7), the prediction was that fauna in farm zones nos. 1 and 3 should be at least moderately similar to the fauna of fish farms nos. 4 and 2, respectively. This should be true particularly for farms nos. 2 and 3, which are only about 80 km from each other. However, such a faunal similarity does not occur (Fig. 10). Rather, farm zones nos. 1 and 3 are faunally unrelated to the remaining zones in the study, and also very different from each other. The distinctive character of the fauna under the cages of farm no. 1 is derived from both high abundance of dorvilleid polychaetes and some trochid gastropods, and from particularly low abundance of lucinid and corbulid bivalves and leucothoid amphipods (Fig. 12b). Interestingly, these taxa are unrelated to conditions of organic pollution, the faunal differences between farm no. 1 and its control thus being likely due to differences in sediment and microhabitat features. In contrast, farm zone no. 3 is characterized by a high abundance of capitellid polychaetes and nassarid gastropods, as well as relatively high values of organic matter and bacterial concentration (Fig. 12). These features indicate that fish farm no. 3, which is the most important in fish biomass production out of the five studied fish farms, is the only one exerting a detectable environmental impact on the local environment and fauna.
The CCA with variance partitioning revealed that the water-column variables (dissolved nutrients and bacterioplankton) are about two times more determinant of spatial taxa variability than the benthic variables. Nonetheless, the mechanisms through which the effect of the water-column variables is exerted on the benthos remain unclear from the current approach.
References
Ackefors H, Enell M (1994) The release of nutrients and organic matter from aquaculture systems in Nordic countries. J Appl Ichthyol 10:225–241
Alongi DM, Chong VC, Dixon P, Sasekumar A, Tirendi F (2003) The influence of fish cage aquaculture on pelagic carbon flow and water chemistry in tidally dominated mangrove estuaries of peninsular Malaysia. Mar Environ Res 55:313–333
Basurco B (2000) Offshore mariculture in Mediterranean countries. Cah Options Méditerr 30:9–18
Basurco B, Larrazabal G (2000) Marine fish farming in Spain. Cah Options Méditerr 30:45–56
Belias C, Bikas V, Dassenakis M, Scoullos M (2003) Environmental impacts of coastal aquaculture in Eastern Mediterranean bays. The case of Astakos Gulf, Greece. Environ Sci Pollut Res 10:287–295
Black EA (1991) Coastal resource mapping. A Canadian approach to resource allocation for salmon farming. Eur Aquaculture Soc Spec Publ 16:441–459
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Bravo FT, Montañes AS (2001) Aquaculture and environment from the perspective of a Spanish fish farmer. Cah Options Méditerr 55:101–109
Brown JR, Gowen RJ, McLusky DS (1987) The effect of salmon farming on the benthos of a Scottish sea loch. J Exp Mar Biol Ecol 109:39–51
Del Giorgio A, Bird DF, Prairie YT, Planas D (1996) Flow citometric determination of bacterial abundance in lake plankton with the green nucleic acid stain SYTO 13. Limnol Oceanogr 41:783–789
Delgado O, Grau A, Pou S, Riera F, Massuti C, Zabala M, Ballesteros E (1997) Seagrass regression caused by fish cultures in Fornell Bay (Menorca, Western Mediterranean). Ocean Acta 20:557–563
Delgado O, Ruiz JM, Pérez M, Romero J, Ballesteros E (1999) Effects of fish farming on seagrass (Posidonia oceanica) in a Mediterranean bay: seagrass decline after organic loading cessation. Ocean Acta 22:109–117
Dempster T, Sanchez-Jerez P, Bayle-Sempere JT, Giménez-Casalduero F, Valle C (2002) Attraction of wild fish to sea-cage fish farms in the south-western Mediterranean Sea: spatial and short-term temporal variability. Mar Ecol Progr Ser 242:237–252
Dosdat A (2001) Environmental impact of aquaculture in the Mediterranean: nutritional and feeding aspects. Cah Options Méditerr 55:23–36
Fabiano M, Danovaro R, Fraschetti S (1995) A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the Ligurian Sea (northwestern Mediterranean). Cont Shelf Res 15:1453–1469
Findlay RH, Watling L (1994) Towards a process level model to predict the effects of salmon net pen aquaculture on the benthos. Can Tech Rep Fish Aquat Sci 1949:47–77
Gasol JM, Del Giorgio A (2000) Using flow citometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Sci Mar 64:197–224
GESAMP (1996) Monitoring the ecological effects of coastal aquaculture wastes. Rep Stud GESAMP 57:1–38
Gowen RJ (1994) Managing eutrophication associated with aquaculture development. J Appl Ichthyol 10:242–257
Gowen RJ, Ezzi IA (1992) Assessment and prediction of the potential for hypernutrification and eutrophication associated with caged culture of salmonids in Scottish coastal waters. Natural Environment Research Council, Oban, Scotland
Hall POJ, Holby O, Kollberg S, Samuelsson MO (1992) Chemical fluxes and mass balance in a marine fish cage farm. IV. Nitrogen. Mar Ecol Progr Ser 89:81–91
Hargrave BT, Kress N, Brenner S, Gordon LI (1991) Phosphorous limitation of primary productivity in the eastern Mediterranean Sea. Limnol Oceanogr 36:424–432
Hargrave BT, Duplisea DE, Pfeiffer E, Wildish DJ (1993) Seasonal changes in benthic fluxes of dissolved oxygen and ammonium associated with marine cultured Atlantic salmon. Mar Ecol Progr Ser 96:249–257
Hargrave BT, Phillips GA, Doucette LI, White MJ, Illigan TG, Ildish DJ, Ranston RE (1997) Assensing benthic impacts of organic enrichment from marine aquaculture. Water Air Soil Pollut 99:641–650
Karakassis I (2001) Ecological effects of fish farming in the Mediterranean. Cah Options Méditerr 55:15–22
Karakassis I, Hatziyanni E (2000) Benthic disturbance due to fish farming analyzed under different levels of taxonomic resolution. Mar Ecol Progr Ser 184:205–218
Karakassis I, Tsapakis M, Hatziyanni E (1998) Seasonal variability in sediment profiles beneath fish farm cages in the Mediterranean. Mar Ecol Progr Ser 162:243–252
Karakassis I, Hatziyanni E, Tsapakis M, Plaiti W (1999) Benthic recovery following cessation of fish farming: a series of successes and catastrophes. Mar Ecol Progr Ser 184:205–218
Karakassis I, Tsapakis M, Hatziyanni E, Papadopoulou K-N, Plaiti W (2000) Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J Mar Sci 57:1462–1471
Karakassis I, Tsapakis M, Hatziyanni E, Pitta P (2001) Diel variation of nutrients and chlorophyll in sea bream and sea bass cages in the Mediterranean. Fresenius Environ Bull 10:278–283
Krom MD, Kress N, Brenner S, Gordon LI (1991) Phosphorous limitation of primary productivity in the eastern Mediterranean Sea. Limnol Oceanogr 36:424–432
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Lorenzen CJ (1966) A method for the continous measurement of in vivo chlorophyll concentration. Deep-Sea Res 13:223–227
Marie D, Vaulot D, Paternsky F (1996) Application of the novel nucleic acid dyes YOYO-1,YO-PRO-1 and picogreen for flow cytometric analysis of marine prokaryotes. Appl Environ Microbial 62:1649–1655
Mazzola A, Mirto S, Danovaro R (1999) Initial fish-farm impact on meiofaunal assemblages in coastal sediments of the western Mediterranean. Mar Pollut Bull 38:1126–1133
Mazzola A, Mirto S, La Rosa T, Fabiano M, Danovaro R (2000) Fish-farming effects on benthic community structure in coastal sediments: analysis of meiofaunal recovery. ICES J Mar Sci 57:361–379
Mirto S, La Rosa T, Danovaro R, Mazzola A (2000) Microbial and meiofaunal response to intensive mussel-farm biodeposition in coastal sediment of the Western Mediterranean. Mar Pollut Bull 40:244–252
Molina L, López G, Vergara JM, Robaina L (2001) A comparative study of sediments under a marine cage farm at Gran Canaria Island (Spain). Preliminary results. Aquacultur 192:225–231
Persson G (1991) Eutrophication resulting from salmonid fish culture in fresh and salt waters: Scandinavian experiences. In: Cowey CB, Cho CY (eds) Nutritional strategies and aquaculture waste. In: Proceedings of the first international symposium on nutritional strategies in management of aquaculture waste, University of Guelph, Ontario, pp 163–185
Pielou EC (1969) An introduction to mathematical ecology. Wiley, New York
Pitta P, Karakassis I, Tsapakis M, Zivanovic S (1999) Natural versus mariculture induced variability in nutrients and plankton in the eastern Mediterranean. Hydrobiologia 391:181–194
Ritz DA, Lewis ME, Shen M (1990) Response to organic enrichment of infaunal macrobenthic communities under salmonid sea cages. Mar Biol 103:211–214
Ruiz JM, Pérez M, Romero J (2001) Effects of fish farm loadings on seagrass (Posidonia oceanica) distribution, growth and photosynthesis. Mar Pollut Bull 42:749–760
Tsutsumi H, Kikuchi T (1983) Benthic ecology of a small cove with seasonal oxygen depletion caused by organic pollution. Publ Amakusa Mar Biol Lab 7:17–40
Vezzulli L, Chelossi E, Riccardi G, Fabiano M (2002) Bacterial community structure and activity in fish farm sediments on the Ligurian sea (Western Mediterranean). Aquac Int 10:123–141
Vita R, Marín A, Madrid JA, Jiménez-Brinquis B, Cesar A, Marín-Guirao L (2004) Effects of wild fishes on waste exportation from a Mediterranean fish farm. Mar Ecol Prog Ser 277:253–261
Wentworth W (1972) A scale of grade and class terms for clastic sediments. J Geol 30:377–389
Wu RSS (1995) The environmental impact of marine fish culture: towards a sustainable future. Mar Pollut Bull 31:159–166
Wu RSS, Lam KS, Mackay DW, Lau TC, Yam V (1994) Impact of marine fish farming on water quality and bottom sediment: a case studies in the sub-tropical environment. Mar Environ Res 38:115–145
Acknowledgements
We thank J. Gasol and E. Blanc for help with cytometry, S. Pla for help with nutrient analysis, A. Mateos and C. Hermoso for coordinating sampling, and J. Gil for help with polychaete taxonomy. We also thank the fish farming companies that kindly made this study possible and opted to remain anonymous. These investigations have partially benefited from funds from two grant to M.M. (BMC-2002- 01228; PROFIT-310100-2004-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.-D. Franke
Rights and permissions
About this article
Cite this article
Maldonado, M., Carmona, M.C., Echeverría, Y. et al. The environmental impact of Mediterranean cage fish farms at semi-exposed locations: does it need a re-assessment?. Helgol Mar Res 59, 121–135 (2005). https://doi.org/10.1007/s10152-004-0211-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-004-0211-5