Abstract
Four patients with rectal cancer required reconstruction of a defect of the posterior vaginal wall. All patients received neoadjuvant (chemo)radiotherapy, followed by an en bloc (abdomino)perineal resection of the rectum and posterior vaginal wall. The extent of the vaginal defect necessitated closure using a tissue flap with skin island. The gluteal turnover flap was used for this purpose as an alternative to conventional more invasive myocutaneous flaps (gracilis, gluteus, or rectus abdominis). The gluteal turnover flap was created through a curved incision at a maximum width of 2.5 cm from the edge of the perineal wound, thereby creating a half-moon shape skin island. The subcutaneous fat was dissected toward the gluteal muscle, and the gluteal fascia was incised. Thereafter, the flap was rotated into the defect and the skin island was sutured into the vaginal wall defect. The contralateral subcutaneous fat was mobilized for perineal closure in the midline, after which no donor site was visible.The duration of surgery varied from 77 to 392 min, and the hospital stay ranged between 3 and 16 days. A perineal wound dehiscence occurred in two patients, requiring an additional VY gluteal plasty in one patient. Complete vaginal and perineal wound healing was achieved in all patients. The gluteal turnover flap is a promising least invasive technique to reconstruct posterior vaginal wall defects after abdominoperineal resection for rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In locally advanced or recurrent patients with rectal cancer, the tumor may invade the posterior vaginal wall. Following the oncological principles of en bloc resection, an abdominoperineal resection (APR) with partial or complete excision of the posterior vaginal wall is often necessary, even after neoadjuvant chemoradiotherapy. For small defects in the posterior vaginal wall, primary closure may be an option, although this has the risk of dehiscence or narrowing, especially after radiotherapy. Larger vaginal wall defects have to be closed with well-vascularized tissue, to enable healing of the vagina as well as the adjacent perineal wound, thereby minimizing the risk of chronic pelviperineal abscess and fistula formation.
To date, myocutaneous flaps, such as the gracilis flap, rectus abdominal muscle (RAM) flap, gluteal maximus flap, and fasciocutaneous gluteal perforator flaps, are the most frequently described autologous tissue flaps to reconstruct the posterior vaginal wall and close the perineum [1,2,3,4,5,6,7]. However, these flaps require extensive dissection with associated risks of donor site morbidity, and pedicled flaps might even fail [2, 8]. There is a need for less invasive reconstructive alternatives to reduce the morbidity after extensive pelvic resections involving the posterior vaginal wall.
The gluteal turnover flap comprises the neighboring skin and subcutaneous tissue of one buttock, which is perfused by vessels perforating the superficial fascia of the gluteus muscle. It involves minimal dissection, carries a negligible risk of flap failure, and enables midline closure without donor site scar [9]. The gluteal turnover flap can be used for perineal closure during primary abdominoperineal resection, salvage surgery for chronic pelvic sepsis, and perineal hernia repair [9,10,11,12,13]. Recently, we extended the indication by using the skin island of the gluteal turnover flap to close a posterior vaginal wall defect. A similar flap has been described as vaginal reconstructive technique in a case report previously [14]. The aim of this technical note is to present a comprehensive description, along with images, of this innovative reconstruction technique and outcomes in four patients.
Materials and methods
Patients
From March 2021 until November 2023, patients who underwent an APR for primary or locally recurrent rectal cancer and whose tumor invaded the posterior vaginal wall requiring an en bloc resection were identified. Exclusion criteria were closure of the posterior vaginal wall defect by primary closure or by the use of a flap other than the gluteal turnover flap. Patients were included after obtaining written informed consent.
Data collection
Baseline characteristics and medical history were obtained from patients’ records. Procedural characteristics of index surgery, complications, reinterventions, hospital stay, and follow-up were collected. Successful posterior vaginal wall reconstruction using a gluteal turnover flap was defined as complete healing of the perineum and the vaginal wall, without any signs of an abscess or fistula.
Surgical technique
Vaginal wall reconstruction using gluteal turnover flap
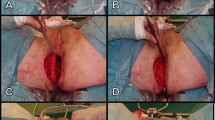
All patients received prophylactic intravenous antibiotics and were positioned in lithotomy position (Fig. 1).
Patients underwent an (extralevatoir) APR with en bloc resection of a major part of the posterior vaginal wall (Fig. 2).
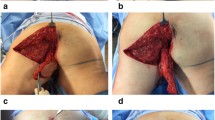
A half-moon shaped skin island with a maximum of 2.5 cm of adjacent skin on either the left or right buttock side is marked (Fig. 3a). After the skin is incised, a flap is created by dissecting the subcutaneous fat toward the gluteal muscle at an angle of 45°, with an evenly 2–3 cm thickness of the flap (Fig. 3b).
The skin flap is turned inwards with the most caudal point toward the proximal edge of the vaginal defect (Fig. 4).
Positioning of the gluteal turnover flap. a Caudal end of the skin is moved toward the proximal edge of the vaginal defect (Fig. 2, arrow 2). b The flap is hold in place manually to mark the skin
The part of the skin island needed for tensionless closure of the posterior vaginal wall defect is determined and marked on the flap (Fig. 5a). If needed, the flap is partially de-epithelialized to fit the vaginal wall defect (Fig. 5b).
Defect closure is started with placement of interrupted Vicryl 3.0 sutures at the level of the posterior vaginal fornix (Fig. 6a, b). Then, the gluteal skin island is fixated bilaterally using continuous Vicryl 3.0 sutures and lastly to the distal posterior vaginal wall/perineal body (Fig. 6c) or, in case of posterior vaginal wall resection including the perineal body, to the perineal skin.
Next, the subcutaneous part of the flap is fixed to the pelvic floor remnants and ischioanal fat. In this position, the flap fills the noncollapsible dead space of the previous anal canal and supports the neovagina in its anatomical position (Fig. 7a). The contralateral subcutaneous fat is dissected over the gluteus muscle to enable tensionless midline closure. Subcutaneous fat and skin are closed in layers over a 14 French vacuum drain, which is positioned on the flap (Fig. 7b).
Closure of the subcutaneous fat and skin in midline. a (Sub)cutaneous perineal defect after fixation of the skin island to the vaginal wall and fixation of the subcutaneous part of the flap at the deepest point of the cavity. b Final situation with a drain (*) in the space between the flap and subcutaneous fat
After the surgery, there are no postoperative restrictions regarding sitting or mobilization for patients. The vacuum drain is kept for a minimum of 5 days and until the production is < 10 cc/24 h.
Results
Baseline and surgical characteristics
Between January 2021 until December 2023, five patients were retrospectively identified, of which one patient did not sign informed consent. Three of the four remaining patients were diagnosed with primary rectal cancer and underwent neoadjuvant (chemo)radiotherapy (Table 1) followed by APR. The fourth patient developed a recurrent rectal carcinoma for which neoadjuvant rechemoradiotherapy was given, and a transperineal resection of the rectal stump was performed. There were no intraoperative complications.
Postoperative course and follow up
In the first patient, a superficial perineal wound dehiscence occurred, which had completely closed through secondary wound healing after 91 days. The second patient visited the emergency room six days after discharge because of vaginal blood loss from a minor defect of the posterior vaginal wall. No readmission nor reintervention was needed, and after 97 days, the posterior vaginal wall was completely healed as well as the perineum. The third patient was readmitted because of a perineal wound infection six days after being discharged. The entire length of the perineal wound had dehisced and the small bowel was exposed at the level of the sacrum. The gluteal turnover flap was viable and the reconstructed posterior vaginal wall was intact. An additional contralateral VY fasciocutaneous gluteal plasty was performed. After 108 days, the perineum was completely healed. The postoperative course of the last patient was uncomplicated, and complete wound healing was achieved after 31 days.
At the time of assessment, patient one and three are still without long-term complications. The second patient has a permanent catheter because of urinary incontinence. No perineal hernia or fistula formation was observed in any of the four patients.
Discussion
The current article describes an innovative least invasive surgical technique to close a defect in the posterior vaginal wall following extensive resection of locally advanced or recurrent rectal cancer. This technique supplements existing closure methods such as primary closure, gracilis flap, RAM flap, and other gluteal perforator flaps [1,2,3, 15].
The main advantage of the gluteal turnover flap for this indication is the very little additional dissection needed for flap harvesting and the absence of a donor site scar. The main disadvantage is related to the use of adjacent perineal tissue that can interfere with tensionless closure, with inherent risk of wound healing problems. In the four presented patients, it took 1−4 months to achieve complete wound healing. A major risk factor contributing to this is neoadjuvant radiotherapy [16]. However, the donor site area of a gluteal turnover flap has often been outside the radiation field in patients with rectal cancer. Furthermore, a perineal wound dehiscence was seen in two patients, which consequently prolonged healing time. Based on our experience with these flaps, a wound dehiscence often heals by secondary intention without long-term consequences but might sometimes require vacuum-assisted closure techniques or surgical reintervention.
Compared with gracilis and RAM flaps, a major advantage of the flaps originating from the gluteal region is the limitation of donor site morbidity. Using a gluteal fasciocutaneous perforator flap, the donor site is generally not associated with major complications and is preferable to an abdominal wall donor site with risk of hernia formation, or inner thigh with a large visible scar [15]. There is even no additional scar by using a gluteal turnover flap, and there is no need for restrictions regarding sitting or walking in the direct postoperative period. Also, the vascularization of this flap is reliable due to the abundance of perforators without the need of Doppler ultrasound or indocyanine green-enhanced fluorescence [17]. Finally, the gluteal turnover flap is a simple technique that can be performed by a colorectal surgeon, eliminating the need for a plastic surgeon.
In case of more extended resections with requirement of more tissue bulk, the gluteal turnover flap is not sufficient. In this case, a uni- or bilateral gluteal VY fasciocutaneous flap might be an alternative [5, 18]. Another limitation of the gluteal turnover flap might be the radiation-induced impaired condition of the perineal skin, which makes it generally not suitable in patients undergoing APR for residual or recurrent anal cancer after primary radiotherapy.
Conclusions
The gluteal turnover flap seems to be an attractive reconstructive technique to close a defect in the posterior vaginal wall after APR for rectal cancer, with several advantages related to limited additional dissection for flap harvesting.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kaartinen IS, Vuento MH, Hyöty MK, Kallio J, Kuokkanen HO (2015) Reconstruction of the pelvic floor and the vagina after total pelvic exenteration using the transverse musculocutaneous gracilis flap. J Plast Reconstr Aesthet Surg 68(1):93–97
Radwan RW, Tang AM, Harries RL, Davies EG, Drew P, Evans MD (2021) Vertical rectus abdominis flap (VRAM) for perineal reconstruction following pelvic surgery: A systematic review. J Plast Reconstr Aesthet Surg 74(3):523–529
Coltro PS, Busnardo FF, Mônaco Filho FC, Olivan MV, Millan LS, Grillo VA et al (2017) Outcomes of immediate internal pudendal artery perforator flap reconstruction for irradiated abdominoperineal resection defects. Dis Colon Rectum 60(9):945–953
Assi H, Persson A, Palmquist I, Öberg M, Buchwald P, Lydrup ML (2022) Short-term outcomes following beyond total mesorectal excision and reconstruction using myocutaneous flaps: a retrospective cohort study. Eur J Surg Oncol 48(5):1161–1166
Pappou E, Ben-Yaakov A, Jiménez-Rodríguez RM, Garcia-Aguilar J (2024) Simultaneous posterior vaginal and perineal reconstruction using gluteal fasciocutaneous flaps following pelvic exenteration with sacrectomy. Br J Surg. https://doi.org/10.1093/bjs/znad395
Bolmstrand B, Sommar P, Nilsson PJ, Zach D, Lagergren J, Schain D et al (2022) Vaginal reconstruction using a gluteal transposition flap after abdominoperineal excision for anorectal malignancy. Updates Surg 74(2):467–478
Hellinga J, Khoe PC, van Etten B, Hemmer PH, Havenga K, Stenekes MW, Eltahir Y (2016) Fasciocutaneous lotus petal flap for perineal wound reconstruction after extralevator abdominoperineal excision: application for reconstruction of the pelvic floor and creation of a neovagina. Ann Surg Oncol 23(12):4073–4079
Kim E, Fernando C, McCombie A, Bailey W, Frizelle F, Glyn T et al (2022) Abdominal and perineal hernia rates following vertical rectus abdominis myocutaneous (VRAM) flap reconstruction—a supraregional experience. J Plast Reconstr Aesthet Surg 75(3):1158–1163
Kreisel SI, Sparenberg S, Sharabiany S, Hompes R, Lapid O, van der Horst C et al (2023) Gluteal fasciocutaneous flap reconstruction after salvage surgery for pelvic sepsis. Dis Colon Rectum 66:1570–1577
Sharabiany S, Brouwer TPA, Kreisel SI, Musters GD, Blok RD, Hompes R, Tanis PJ (2022) Mesh, flap or combined repair of perineal hernia after abdominoperineal resection—a systematic review and meta-analysis. Colorectal Dis 24(11):1285–1294
Sharabiany S, Blok RD, Lapid O, Hompes R, Bemelman WA, Alberts VP et al (2020) Perineal wound closure using gluteal turnover flap or primary closure after abdominoperineal resection for rectal cancer: study protocol of a randomised controlled multicentre trial (BIOPEX-2 study). BMC Surg 20(1):164
Sharabiany S, van Dam JJW, Sparenberg S, Blok RD, Singh B, Chaudhri S et al (2021) A comparative multicentre study evaluating gluteal turnover flap for wound closure after abdominoperineal resection for rectal cancer. Tech Coloproctol 25(10):1123–1132
Chasapi M, Maher M, Mitchell P, Dalal M (2018) The perineal turnover perforator flap: a new and simple technique for perineal reconstruction after extralevator abdominoperineal excision. Ann Plast Surg 80(4):395–399
Moura FS, Chasapi M, Mitchell P, Dalal MD (2021) Perineal turn over perforator flap: a novel surgical technique for combined perineal and posterior vaginal wall reconstruction. World J Plast Surg 10(1):114–118
Smeets L, Hendrickx B, Teo TC (2012) The propeller flap concept used in vaginal wall reconstruction. J Plast Reconstr Aesthet Surg 65(5):629–633
Chadwick MA, Vieten D, Pettitt E, Dixon AR, Roe AM (2006) Short course preoperative radiotherapy is the single most important risk factor for perineal wound complications after abdominoperineal excision of the rectum. Colorectal Dis 8(9):756–761
Blok RD, Hagemans JAW, Burger JWA, Rothbarth J, van der Bilt JDW, Lapid O et al (2019) Feasibility of a subcutaneous gluteal turnover flap without donor site scar for perineal closure after abdominoperineal resection for rectal cancer. Tech Coloproctol 23(8):751–759
Gielen AHC, Colier E, Qiu SS, Keymeulen K, Stassen LPS, Melenhorst J (2023) Research highlight: surgical outcomes of gluteal VY plasty after extensive abdominoperineal resection or total pelvic exenteration. Langenbecks Arch Surg 408(1):157
Funding
No funding has been received by any author in relation to this article.
Author information
Authors and Affiliations
Contributions
S.K., R.C., and G.D. wrote the main manuscript text, and P.T. and J.R. provided and prepared Figs. 1−7. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors declare a conflict of interest.
Ethical approval
Ethical approval was obtained from the ethical committee from the Erasmus MC.
Informed consent
Written informed consent was obtained from all participating patients, including explicit consent to publish images.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kreisel, S.I., van den Braak, R.R.J.C., Rothbarth, J. et al. Introducing an innovative surgical technique: gluteal turnover flap for posterior vaginal wall reconstruction: a case series. Tech Coloproctol 28, 70 (2024). https://doi.org/10.1007/s10151-024-02941-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10151-024-02941-3