Abstract
Purpose
Sarcopenia is associated with poor short- and long-term patient outcomes following colorectal surgery. Despite postoperative ileus (POI) being a major complication following colorectal surgery, the predictive value of sarcopenia for POI is unclear. We assessed the association between sarcopenia and POI in patients with colorectal cancer.
Methods
Elective colorectal cancer surgery patients were retrospectively included (2018–2022). The cross-sectional psoas area was calculated using preoperative staging imaging at the level of the 3rd lumbar vertebrae. Sarcopenia was determined using gender-specific cut-offs. The primary outcome POI was defined as not achieving GI-2 by day 4. Demographics, operative characteristics, and complications were compared via univariate and multivariate analyses.
Results
Of 297 patients, 67 (22.6%) were sarcopenic. Patients with sarcopenia were older (median 74 (IQR 67–82) vs. 69 (58–76) years, p < 0.001) and had lower body mass index (median 24.4 (IQR 22.2–28.6) vs. 28.8 (24.9–31.9) kg/m2, p < 0.001). POI was significantly more prevalent in patients with sarcopenia (41.8% vs. 26.5%, p = 0.016). Overall rate of complications (85.1% vs. 68.3%, p = 0.007), Calvien-Dindo grade > 3 (13.4% vs. 10.0%, p = 0.026) and length of stay were increased in patients with sarcopenia (median 7 (IQR 5–12) vs. 6 (4–8) days, p = 0.013). Anastomotic leak rate was higher in patients with sarcopenia although the difference was not statistically significant (7.5% vs. 2.6%, p = 0.064). Multivariate analysis demonstrated sarcopenia (OR 2.0, 95% CI 1.1–3.8), male sex (OR 1.9, 95% CI 1.0–3.5), postoperative hypokalemia (OR 3.2, 95% CI 1.6–6.5) and increased opioid use (OR 2.4, 95% CI 1.3–4.3) were predictive of POI.
Conclusion
Sarcopenia demonstrates an association with POI. Future research towards truly identifying the predictive value of sarcopenia for postoperative complications could improve informed consent and operative planning for surgical patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia has become increasingly recognized as a significant contributor to poor short- and long-term patient outcomes following colorectal surgery [1,2,3,4]. Sarcopenia is the development of frailty associated with advanced aging and results in a reduction of muscle mass and/or function [5, 6]. Patients with colorectal malignancy have sarcopenia compounded as a result of the pronounced effects of malignancy on metabolism, and neoadjuvant therapy resulting in reduced appetite, vomiting and pain [5, 6]. In a recent meta-analysis, 37% of patients with colorectal cancer had sarcopenia, and this was associated with an increase in severe postoperative complications and mortality [3]. As preoperative staging computed tomography (CT) is routinely performed, the cross-sectional area of the psoas can be easily determined to quantify lean muscle mass, offering an opportunity to diagnose and to potentially intervene in high-risk patients with sarcopenia [7, 8].
Postoperative ileus (POI) occurs in up to 25% of cases following colorectal resection [9,10,11]. With paralysis of the gastrointestinal (GI) tract, patients experience abdominal distention, diet intolerance, constipation, nausea, and vomiting, increasing risk of complications such as pneumonia and delayed wound healing [12, 13]. Furthermore, POI increases the risk of renal and hepatic failure, prolongs hospital stay, and increases 30-day readmission [12, 13]. As a result of this POI poses a significant financial burden to patients and healthcare services, increasing total hospital costs by 26% in Australia and 50–100% worldwide [14,15,16,17].
Despite the high prevalence of preoperative sarcopenia and POI as a complication in colorectal surgical patients, there is limited literature establishing a connection between them. POI is well known to be associated with advanced age and significant comorbidities [18]. Similarly, sarcopenia has a strong link to geriatric syndromes and constipation [19]. It has also been speculated that diet imbalances related to sarcopenia impair macrophage response to peritoneal irritation, resulting in POI [20]. Trejo-Avila et al. identified 23 papers investigating postoperative colorectal complications in patients with sarcopenia; however, only four articles reported POI as a complication [21,22,23,24]. These studies did not define POI, had low sample sizes and reported a disproportionately low incidence of POI in a colorectal cohort where POI is expectedly common. Of these studies, one demonstrated a higher incidence of POI in the sarcopenic group; however, the assessment was limited by sample size (25% vs. 17.4%, n = 47) [23]. Noting the lack of literature on the predictive value of sarcopenia, Sasaki et al. investigated the correlation between skeletal muscle index (SMI) and POI [25]. Using bioelectrical impedance analysis to quantify SMI, they found that low SMI was associated with an increased likelihood of developing POI (HR 10.8 (95% CI 1.25–93.20), p = 0.031), independent of age and sex. There remains a significant gap in the literature investigating the incidence of POI and the return of gastrointestinal function using validated composite measures and strict clinical definitions in patients with sarcopenia following colorectal surgery.
This study aims to assess the role of sarcopenia as a predictor for POI following colorectal surgery.
Methods
This study was approved by the Central Adelaide Local Health Network (CALHN) Human Research Ethics Committee with a waiver of consent for retrospective studies and is reported using the Strengthening The Reporting of Observational studies in Epidemiology (STROBE) guidelines [26].
Patient selection
This study was performed at the Colorectal Unit of the Royal Adelaide Hospital (RAH), a tertiary referral centre in South Australia, Australia. Patients who underwent elective colorectal cancer surgery between January 2018 and June 2022 were identified through the prospective colorectal cancer database of patients discussed at the weekly RAH Colorectal Cancer multidisciplinary meeting. All patients at the RAH are placed on an enhanced recovery pathway (ERP) postoperatively. The ERP protocol can be found at www.tinyurl.com/raheras.
Inclusion and exclusion criteria
Consecutive elective colorectal patients over 18 years old who underwent major bowel surgery, consisting of large bowel resection for colorectal malignancy, were included. Patients were excluded if they had undergone emergency surgery, small bowel resection, defunctioning ostomy without bowel resection, colonic stenting, transanal endoscopic microsurgery (TEMS), care was delivered at a different facility or had non-operative management. Patients were also excluded if they had insufficient data or had not undergone staging CT accessible by using our local Picture Archiving and Communication System (PACS) or InteleViewer™ Australia.
Sarcopenia calculation
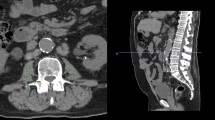
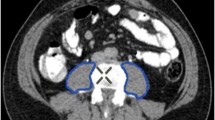
Lean muscle mass was calculated using the protocol defined by Jones et al. using preoperative staging CT scans [8]. Staging CT scans were retrieved from the time of diagnosis or post neoadjuvant therapy prior to operation, whichever came later. Total psoas area (TPA) was calculated by multiplying the longest anterior–posterior (AP) and transverse muscle diameters bilaterally (Fig. 1). Measurements were taken at the level of the third lumbar vertebrae using PACS or InteleViewer™ Australia. This method of AP and transverse muscle diameter calculation has been proven comparable to computer-calculated TPA (r2 0.944 (95% CI 0.86–0.97), p = 0.001) [8]. TPA was normalized for the patient’s height squared (TPAmm2/m2) to calculate the total psoas area index (TPAI). Sarcopenia was defined using previously validated gender-specific cut-off points: < 385 mm2/m2 in women and < 545 mm2/m2 in men [27]. Two investigators (LT and SB) independently calculated lean muscle mass for all patients to identify sarcopenia. Conflicting results were resolved by consensus.
Data collection
Medical records were reviewed retrospectively. Known risk factors for the development of POI were collected [9,10,11, 14, 28]. Baseline demographics included age, gender, height, weight, body mass index (BMI), American Society of Anesthesiologists (ASA) score, American Joint Committee on Cancer (AJCC) stage and preoperative neoadjuvant treatment. Components of the modified frailty index (MFI)-5 (i.e. patient functional status, and medical history of congestive cardiac failure, chronic obstructive pulmonary disease, diabetes mellitus and hypertension requiring medication) were collated as the MFI-5 has been associated with frailty and poor surgical outcomes following colorectal surgery [4]. Other nutritional data, including total protein and albumin, were also collected. Operative data included surgical approach (open/laparoscopic), conversion rates, procedure type, stoma formation, duration of surgery, and perioperative intravenous fluid administration. Postoperative data included requirements in morphine equivalents (intraoperative, postoperative recovery and day 1–4) calculated using Opioid Calculator v2.9.1 (Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists, Australia).
Outcomes
The primary outcome was POI, defined by not achieving GI-2 by postoperative day 4, a threshold suggested by Vather et al. [29]. GI-2 is a validated outcome measure comprising time to first stool and tolerance of solid diet without significant nausea or vomiting [30]. GI-2 was determined by a retrospective review of daily entries by medical and nursing staff. Patients discharged prior to achieving GI-2 were considered to not have POI. Secondary outcomes included time to first stool, time to tolerance of oral diet, nasogastric tube (NGT) reinsertion and complications for both groups. Length of stay and 30-day complications (Clavien-Dindo grade, CD; Comprehensive complication index, CCI) were recorded [31, 32].
Statistical methods
Statistical analysis was performed using SPSS 28.0 (SPSS Inc., Armonk, NY, USA). Numerical data are presented as median (IQR [range]) or mean (standard deviation) depending on parametricity identified with the Shapiro–Wilk test. Univariate analysis was performed using the Mann–Whitney U test for nonparametric variables or the Student t test for normally distributed continuous variables. The χ2 or Fisher’s exact test (when expected n < 5) was applied for categorical variables. All collected variables were used in the univariate logistic regression analysis. Statistically significant variables were then used for multivariate logistic regression analyses to determine predictors of POI. Data for multivariate logistic analyses were evaluated and met all assumptions. P values of less than 0.05 were considered statistically significant.
Results
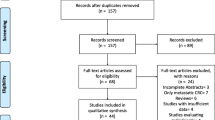
Of 297 patients included in the analysis, 22.6% (n = 67) were diagnosed with sarcopenia. Patient selection is reported in Fig. 2. Baseline characteristics demonstrate that the sarcopenic group was significantly older (74 vs. 68.5 years, p < 0.001) and showed no difference related to sex distribution (Table 1). The sarcopenic cohort has a significantly lower weight (72 vs. 80 kg, p < 0.001) and body mass index (24.4 vs. 28.8 kg/m2, p < 0.001). These patients were generally more comorbid with a greater proportion of ASA grade ≥ 3 (71.6% vs. 54.8%, p = 0.014) and a higher MFI-5 (44.8% vs. 31.7%, p = 0.048). No difference was found between rates of neoadjuvant therapy given between both groups (14.9% vs. 13.0%, p = 0.691). The sarcopenic group had lower preoperative hemoglobin, total protein and albumin levels (125 vs. 133.5, 71 vs. 73 and 35 vs. 37 g/L, respectively all p < 0.05). Table 2 demonstrates the perioperative features of the two groups. They shared similar surgical characteristics with respect to tumour site, AJCC stage, operative type or approach, and stoma formation or type. There were also no differences in intraoperative administration of opioid and intravenous fluid, or postoperative serum potassium.
Postoperative outcomes are summarized in Table 3. POI rates (41.8% vs. 26.5%, p = 0.016) and NGT reinsertion rates were significantly higher in the sarcopenic group compared to the non-sarcopenic group (38.8% vs. 23.9%, p = 0.016). Median time to tolerance of oral diet (3 vs. 2 days, p = 0.047), first stool (4 vs. 3 days, p = 0.096), and GI-2 (4 vs. 3 days, p = 0.206) was prolonged by 1 day in the sarcopenic group. However, only tolerance to oral diet was found to be statistically significant. Overall complications were greater in the sarcopenic group. In addition to more frequent complications (85.1% vs. 68.3%, p = 0.007), patients with sarcopenia also had higher-grade complications (CD grade 3–5, 13.4% vs. 10.0%, p = 0.026 and CCI 22.6 vs. 12.2, p = 0.007). The sarcopenic cohort were more likely to have more than one complication; however, this did not reach significance (64.9% vs. 56.1%, p = 0.245). Following POI, patients with sarcopenia were more likely to have respiratory complications (20.9% vs. 11.3%, p = 0.043) and urinary tract infections (9.0% vs. 3.0%, p = 0.037). Anastomotic leak rate was higher in patients with sarcopenia although the difference was not statistically significant (7.5% vs. 2.6%, p = 0.064). Other complications such as intensive care unit admission were similar between the two cohorts. The 30-day mortality was low in both cohorts (1.5% vs. 0.8%, p = 0.537). A 1-day median increase in length of stay was seen in patients with sarcopenia (7 vs. 6 days, p = 0.013).
Following univariate analysis, shown in Table 4, several factors were predictive of POI including sarcopenia, male sex, ASA grade ≥ 3, smoking history, previous abdominal surgery, postoperative hypokalemia, open surgical approach, postoperative day 1–4 opioid use greater than median, intensive care unit admission and CD grade ≥ 3. However, on multivariate analysis, sarcopenia (OR 2.0, 95% CI 1.1–3.8, p = 0.029), male sex (OR 1.9, 95% CI 1.0–3.5, p = 0.036), postoperative hypokalemia (OR 3.2, 95% CI 1.6–6.5, p = 0.001) and postoperative opioid use remained strongly predictive of POI (OR 2.4, 95% CI 1.3–4.3, p = 0.004).
Discussion
Our results confirm that in our cohort of colorectal surgical patient’s sarcopenia is associated with increased morbidity, consistent with previously reported data in the literature. However, our study demonstrates a strong association between preoperative radiologically diagnosed sarcopenia and POI by validated criteria following colorectal surgery. Of our patients with newly diagnosed colorectal cancer, 22.6% were found to have sarcopenia. This is similar to our previously reported cohort of patients with locally advanced rectal cancer [3, 33]. Although our rate is lower than published pooled results of 37% found in a recent meta-analysis, this may be explained by other studies using various methods to establish sarcopenia, the inclusion of emergent cases, and variable international ethnicities and demographics.
Overall, we found 41.8% of patients with sarcopenia developed POI. Defining POI is contentious, with no one accepted method. Defining POI using GI-2 has a reported rate of 10.1–34.5%, shown in the literature and in our previous studies [17, 34, 35]. Owing to the variable definitions, earlier studies have not looked at the relationship between POI and sarcopenia in detail. Three papers found a low incidence of postoperative ileus in patients with and without sarcopenia (< 6%) [21, 22, 24], with only one paper demonstrating a higher incidence associated with patients with sarcopenia [23]. The paper closest to our own study, that by Sasaki et al., demonstrated an overall rate of POI of 9.9%, with 19.1% in the sarcopenic cohort developing POI [25]. In our study, the strict definition using a validated composite measure demonstrated a clear correlation between POI and sarcopenia. Our study further corroborates this by the higher requirement for reinsertion of NGT seen in the sarcopenic cohort.
One other colorectal-specific study has investigated the predictive value of a low SMI for POI [25]. Sasaki et al. calculated SMI bioelectrical impedance analysis preoperatively for 213 patients and diagnosed POI using a clinical definition of POI, albeit not a validated measure. Despite using an alternative method of diagnosing low skeletal muscle mass, they demonstrated a difference in the prevalence of POI between low SMI and normal SMI (19.1% vs. 5.5%, p = 0.005). On multivariate analyses for 78 matched patients, SMI remained a predictor of POI (OR 10.80, 95% CI 1.25–93.20, p = 0.031). Our findings along with the results of Sasaki et al. suggest that there is an association between sarcopenia and POI, and that both SMI and TPAI have proven useful prognostic markers for clinicians to identify those at risk of POI.
The association between sarcopenia and POI may be multifactorial. As speculated by Rinaldi et al. [20], patients with sarcopenia have a nutritional imbalance that predisposes them toward POI. Previous studies, including our present study, found sarcopenia is associated with signs of nutritional deficiencies such as decreased hemoglobin levels, serum total protein and albumin [36]. Nutritional imbalance may lead to a pro-inflammatory state, impairing macrophage response to irritation of the peritoneum [20]. This pro-inflammatory state is suspected to contribute to increased complications for patients with sarcopenia [37]. Additionally, smooth muscle contractility is impaired in patients with colorectal cancer as a result of the accumulation of collagen around the intestinal plexus [25]. Furthermore, POI and sarcopenia, separately, have been correlated to elderly and comorbid patients [18, 38, 39]. In our study, despite age and comorbidities being higher in the baseline characteristics for the sarcopenic group, age was not predictive of POI in univariate analyses. ASA grade ≥ 3, only reached significance on the univariate analysis. With sarcopenia being independently predictive of POI, we suggest that an association exists. Besides sarcopenia, male sex, increased opioid use postoperatively, and hypokalemia were also predictive of POI, which is consistent with previous literature [9, 28, 39].
Sarcopenia results in poor short- and long-term postoperative outcomes. In a recent systematic review, patients with colorectal cancer and sarcopenia had an increase in cardiopulmonary complications (OR 2.92, 95% CI 1.96–4.37), severe postoperative complications (OR 1.72, 95% CI 1.10–2.68), and mortality (OR 3.21 95% CI 2.01–5.11) [3]. We also demonstrate that patients with colorectal cancer and sarcopenia had a significant increase in CCI, higher-grade CD complications and respiratory complications. We also found a significant increase in patient’s length of stay. However, this only represented a median increase of 1 day. Interestingly, despite a higher incidence of anastomotic leaks in the sarcopenic group, this did not reach significance. This result is consistent with the pooled results of the meta-analysis; however, in our study, as a result of our sample size and the low overall incidence of anastomotic leaks (3.7%) a type II error cannot be ruled out [3].
Identifying frail patients helps clinicians provide accurate information about the risk of complications to patients as well as guide interventions, such as nutritional support and physiotherapy. Highlighted in this study is TPAI as an easy method to assess colorectal surgical patients. The method of lean muscle mass assessment described by Jones et al. has been shown to be a satisfactory surrogate marker for sarcopenia [3, 8] and allows an easy and convenient measure of lean muscle mass assessment in a cohort of patients that predictably have staging scans available [4, 8]. Assessment of total psoas area represents an easy opportunity to identify patients at risk of postoperative complications, helping clinicians to provide accurate information about surgical risk to their patients. Additionally identifying at-risk patients can prompt clinicians to take early action to treat POI and avoid secondary complications such as respiratory complications or aspiration pneumonia, which was notably higher in the POI group.
Given the results from Sasaki et al. and our findings, methods of modifying sarcopenia and the impact on POI rates could be further explored. However, there are limited reports of prehabilitation programmes that have successfully modified sarcopenia and resulted in improved rates of postoperative complications [40]. To our knowledge, no studies have looked to improve POI rates with prehabilitation, particularly during patients’ neoadjuvant therapy. In a study of colorectal surgical patients undergoing neoadjuvant therapy, a 13- to 17-week guided walking programme improved lean muscle mass [20]. In our study, albeit a small portion of our cohort, 10 of the 13 rectal cancers that were sarcopenic had undergone neoadjuvant therapy. With neoadjuvant therapy now favoured in treating rectal cancers [41], this represents a potential opportunity for prehabilitation to improve postoperative outcomes, like POI, for these patients.
Although demonstrating an association between sarcopenia and POI, this study had several limitations. This study was retrospective and single centred in design. As a result of the limitation of demographic recording, patients in our hospital may declare if they are Aboriginal-Torres Strait Islanders (ATSI) or Non-ATSI. We could not delineate ethnicity further, and the proportion of ATSI patients was too small to make a meaningful analysis. TPAI is used as a marker of sarcopenia, and does not account for myosteatosis, as well as physical tests for sarcopenia. As a result of the paucity of data available, this study was designed as an observational study without a dedicated sample size. Although this study suggests a link between sarcopenia and POI, a prospective multicentred study incorporating physical tests for sarcopenia is required to confirm this positive association.
Conclusion
Sarcopenia demonstrates a significant association with POI and postoperative complications. Future research towards identifying sarcopenia’s impact on complications would improve clinicians' ability to more accurately consent patients for the risk of surgery and assist in operative planning.
Data availability
Not applicable.
Code availability
Not applicable.
References
Richards SJG, Senadeera SC, Frizelle FA (2020) Sarcopenia, as assessed by psoas cross-sectional area, is predictive of adverse postoperative outcomes in patients undergoing colorectal cancer surgery. Dis Colon Rectum 63(6):807–815. https://doi.org/10.1097/DCR.0000000000001633
Sun G, Li Y, Peng Y et al (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33(10):1419–1427. https://doi.org/10.1007/s00384-018-3128-1
Trejo-Avila M, Bozada-Gutierrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M (2021) Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36(6):1077–1096. https://doi.org/10.1007/s00384-021-03839-4
Al-Khamis A, Warner C, Park J et al (2019) Modified frailty index predicts early outcomes after colorectal surgery: an ACS-NSQIP study. Colorectal Dis 21(10):1192–1205. https://doi.org/10.1111/codi.14725
Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N (2020) Sarcopenia in patients with colorectal cancer: a comprehensive review. World J Clin Cases 8(7):1188–1202. https://doi.org/10.12998/wjcc.v8.i7.1188
Pin F, Couch ME, Bonetto A (2018) Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 12(4):420–426. https://doi.org/10.1097/SPC.0000000000000382
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, Mccargar LJ, Baracos VE (2008) A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 33(5):997–1006. https://doi.org/10.1139/H08-075
Jones KI, Doleman B, Scott S, Lund JN, Williams JP (2015) Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 17(1):O20-26. https://doi.org/10.1111/codi.12805
Alhashemi M, Fiore JF, Safa N et al (2019) Incidence and predictors of prolonged postoperative ileus after colorectal surgery in the context of an enhanced recovery pathway. Surg Endosc 33(7):2313–2322. https://doi.org/10.1007/s00464-018-6514-4
Ceretti AP, Maroni N, Longhi M et al (2018) Risk factors for prolonged postoperative ileus in adult patients undergoing elective colorectal surgery: an observational cohort study. Rev Recent Clin Trials 13(4):295–304. https://doi.org/10.2174/1574887113666180521111153
Wolthuis AM, Bislenghi G, Fieuws S, Van Overstraeten ADB, Boeckxstaens G, D’hoore A (2016) Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis 18(1):O1-9. https://doi.org/10.1111/codi.13210
Vather R, Bissett I (2013) Management of prolonged post-operative ileus: evidence-based recommendations. ANZ J Surg 83(5):319–324. https://doi.org/10.1111/ans.12102
Scarborough JE, Schumacher J, Kent KC, Heise CP, Greenberg CC (2017) Associations of specific postoperative complications with outcomes after elective colon resection: a procedure-targeted approach toward surgical quality improvement. JAMA Surg 152(2):e164681. https://doi.org/10.1001/jamasurg.2016.4681
Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S (2015) Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin 31(4):677–686. https://doi.org/10.1185/03007995.2015.1005833
Goldstein JL, Matuszewski KA, Delaney CP et al (2007) Inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. P&T 32(2):82–90
Mao H, Milne TGE, O’grady G, Vather R, Edlin R, Bissett I (2019) Prolonged postoperative ileus significantly increases the cost of inpatient stay for patients undergoing elective colorectal surgery: results of a multivariate analysis of prospective data at a single institution. Dis Colon Rectum 62(5):631–637. https://doi.org/10.1097/DCR.0000000000001301
Traeger L, Koullouros M, Bedrikovetski S et al (2022) Cost of postoperative ileus following colorectal surgery: a cost analysis in the Australian public hospital setting. Colorectal Dis 24(12):1416–1426. https://doi.org/10.1111/codi.16235
Pak H, Maghsoudi LH, Soltanian A, Gholami F (2020) Surgical complications in colorectal cancer patients. Ann Med Surg (Lond) 55:13–18. https://doi.org/10.1016/j.amsu.2020.04.024
Park H, Lim J, Baek JY, Lee E, Jung HW, Jang IY (2021) Status of constipation and its association with sarcopenia in older adults: a population-based cohort study. Int J Environ Res Public Health 18(21):11083. https://doi.org/10.3390/ijerph182111083
Rinaldi JM, Geletzke AK, Phillips BE, Miller J, Dykes TM, Soybel DI (2016) Sarcopenia and sarcopenic obesity in patients with complex abdominal wall hernias. Am J Surg 212(5):903–911. https://doi.org/10.1016/j.amjsurg.2016.03.003
Nakanishi R, Oki E, Sasaki S et al (2018) Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today 48(2):151–157. https://doi.org/10.1007/s00595-017-1564-0
Lee CS, Won DD, Oh SN et al (2020) Prognostic role of pre-sarcopenia and body composition with long-term outcomes in obstructive colorectal cancer: a retrospective cohort study. World J Surg Oncol 18(1):230. https://doi.org/10.1186/s12957-020-02006-3
Jochum SB, Kistner M, Wood EH et al (2019) Is sarcopenia a better predictor of complications than body mass index? Sarcopenia and surgical outcomes in patients with rectal cancer. Colorectal Dis 21(12):1372–1378. https://doi.org/10.1111/codi.14751
Tankel J, Yellinek S, Vainberg E et al (2020) Sarcopenia defined by muscle quality rather than quantity predicts complications following laparoscopic right hemicolectomy. Int J Colorectal Dis 35(1):85–94. https://doi.org/10.1007/s00384-019-03423-x
Sasaki M, Fukuoka T, Shibutani M, Sugimoto A, Maeda K, Ohira M (2022) Usefulness of the skeletal muscle index in postoperative ileus of colorectal cancer patients: a retrospective cohort study. BMC Surg 22(1):448. https://doi.org/10.1186/s12893-022-01887-3
Von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Vather R, Josephson R, Jaung R, Robertson J, Bissett I (2015) Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 157(4):764–773. https://doi.org/10.1016/j.surg.2014.12.005
Vather R, Trivedi S, Bissett I (2013) Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg 17(5):962–972. https://doi.org/10.1007/s11605-013-2148-y
Van Bree SH, Bemelman WA, Hollmann MW et al (2014) Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Ann Surg 259(4):708–714. https://doi.org/10.1097/SLA.0b013e318293ee55
Clavien PA, Barkun J, De Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7. https://doi.org/10.1097/SLA.0b013e318296c732
Bedrikovetski S, Traeger L, Vather R, Sammour T, Moore JW (2022) Does sarcopenia predict local response rates after chemoradiotherapy for locally advanced rectal cancer? Dis Colon Rectum. https://doi.org/10.1097/DCR.0000000000002451
Delaney CP, Marcello PW, Sonoda T, Wise P, Bauer J, Techner L (2010) Gastrointestinal recovery after laparoscopic colectomy: results of a prospective, observational, multicenter study. Surg Endosc 24(3):653–661. https://doi.org/10.1007/s00464-009-0652-7
Dai X, Ge X, Yang J et al (2017) Increased incidence of prolonged ileus after colectomy for inflammatory bowel diseases under ERAS protocol: a cohort analysis. J Surg Res 212(5):86–93. https://doi.org/10.1016/j.jss.2016.12.031
Benedek Z, Todor-Boer S, Kocsis L, Bauer O, Suciu N, Coros MF (2021) Psoas muscle index defined by computer tomography predicts the presence of postoperative complications in colorectal cancer surgery. Medicina (Kaunas). https://doi.org/10.3390/medicina57050472
Reisinger KW, Van Vugt JL, Tegels JJ et al (2015) Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg 261(2):345–352. https://doi.org/10.1097/SLA.0000000000000628
Vather R, Bissett IP (2013) Risk factors for the development of prolonged post-operative ileus following elective colorectal surgery. Int J Colorectal Dis 28(10):1385–1391. https://doi.org/10.1007/s00384-013-1704-y
Chapuis PH, Bokey L, Keshava A et al (2013) Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg 257(5):909–915. https://doi.org/10.1097/SLA.0b013e318268a693
Zhang X, Wang S, Ji W et al (2022) The effect of prehabilitation on the postoperative outcomes of patients undergoing colorectal surgery: a systematic review and meta-analysis. Front Oncol 12:958261. https://doi.org/10.3389/fonc.2022.958261
Garcia-Aguilar J, Patil S, Gollub MJ et al (2022) Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 43(23):2546–2556. https://doi.org/10.1200/JCO.22.00032
Acknowledgements
LT received scholarships from the University of Adelaide Research Training Program Stipend (a1175080) and Royal Australasian College of Surgeons RP Jepson Scholarship.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. LT received scholarships from the University of Adelaide Research Training Program Stipend (a1175080) and Royal Australasian College of Surgeons RP Jepson Scholarship.
Author information
Authors and Affiliations
Contributions
LT: conceptualisation; methodology; investigation; formal analysis; writing original draft. SB: formal analysis, investigation; writing original draft; writing review and editing. TMN: investigation; writing review and editing. YXK: investigation; writing review and editing. ML: supervision; writing review and editing. JM: supervision; writing review and editing. TS: supervision; writing review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Central Adelaide Local Health Network Human Research Ethics Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Traeger, L., Bedrikovetski, S., Nguyen, T. et al. The impact of preoperative sarcopenia on postoperative ileus following colorectal cancer surgery. Tech Coloproctol 27, 1265–1274 (2023). https://doi.org/10.1007/s10151-023-02812-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-023-02812-3