Abstract
Background
Patients with inflammatory bowel disease (IBD) who have had a total colectomy remain with their rectum in situ, and are therefore at risk of rectal carcinoma. It is not clear how high the incidence of rectal cancer is in this cohort. The primary objective of this meta-analysis was to estimate the incidence of rectal cancer in patients with ulcerative colitis or Crohn’s disease who have undergone colectomy but have a residual rectum, and to identify the risk factors for its development. In doing so, we explore the current recommendations for screening processes for these patients.
Methods
A systematic review of the literature was performed. Five databases (Medline, Embase, Pubmed, Cochrane Library and Scopus) were searched from inception to 29 October 2021, to identify studies adhering to the population, intervention, control and outcomes (PICO) criteria. The included studies were critically appraised, and the relevant data was extracted. Cancer incidence was estimated from the reported information. Risk stratification was analysed using RevMan. A narrative approach was undertaken for the exploration of the existing screening guidelines.
Results
Data from 23 of the 24 identified studies was suitable for analysis. The pooled incidence of rectal carcinoma was calculated to be 1.3%. Subgroup analysis showed an incidence of 0.7% and 3.2% for patients with a de-functioned rectal stump and ileorectal anastomosis, respectively. Patients with a history of a colorectal carcinoma were more likely to have a subsequent diagnosis of rectal carcinoma (RR 7.2, 95% CI 2.4–21.1). Patients with previous colorectal dysplasia were also at higher risk (RR 5.1, 95% CI 3.1–8.2). No universal standardised guidance regarding screening for this cohort could be identified in the available literature.
Conclusions
The overall risk of malignancy was estimated to be 1.3%, which is lower than previously reported. There is a need for clear and standardised screening guidance for this group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBD) are conditions with multifaceted, unclear aetiology, and are associated with dysregulation of the immune system that primarily affects the gastrointestinal tract [1, 2]. Long-term complications for IBD patients include an increased risk of colorectal cancer (CRC) [3, 4]
Despite an increasing number of medical therapies [5], surgery remains a mainstay in the management of IBD [6] and 25–35% of patients with IBD will require surgical management during their lifetime [6]. One common procedure in this context is a total abdominal colectomy. After colectomy, the remaining rectum may be stapled off and left in situ or an ileorectal anastomosis (IRA) can be formed.
The British Society of Gastroenterology (BSG) and the Association of Coloproctology of Great Britain and Ireland (ACPGBI) have published comprehensive guidance on bowel surveillance of patients with IBD to detect CRC early, and therefore optimise outcomes and survival [7, 8]. However, this guidance concentrates on patients with an intact colon and there is little available evidence for screening the rectum of patients who have had a colectomy.
The risk of rectal cancer in such patients remains unclear. Previous assessments have estimated the incidence of malignancy to be around 3% [9]. However, this was before current management strategies for IBD were available and it remains unclear if the incidence rate of CRC cancer in IBD patients has changed over time.
The aim of this systematic review and meta-analysis was to provide a synthesis of the available literature to estimate the incidence rate of CRC in patients with a rectal stump after a colectomy for IBD. We also identified risk stratification for such cases and explored the surveillance strategies for the early identification of malignancy.
Materials and methods
Study design
This systematic review and meta-analysis was performed to estimate the incidence of malignancy in the rectal remnant. This study is in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10] and Assessing the methodological quality of systematic reviews (AMSTAR) guidelines [11].
Patient inclusion criteria
The population is patients with a history of IBD who have had a colectomy leaving them with a residual rectal stump or ileorectal anastomosis (IRA). Where appropriate, IBD patients who had a colectomy for CRC or dysplasia were compared with those without either condition. Outcomes were rates of colorectal cancer or descriptors of surveillance regimen published for the early detection of cancer in this setting. Population, intervention, control and outcomes (PICO) criteria are presented in Table 1.
Search strategy
A systematic literature search was performed. Five databases: Medline, Embase, Pubmed, Cochrane Library and Scopus were searched from inception to 29 October 2021. Keywords used in the search terms were ‘Crohn’s disease’, ‘Ulcerative Colitis’, ‘Cancer’, ‘Rectal Stump’ and ‘Ileorectal Anastomosis’. The full search strategy for each database is outlined in Supplementary Table 1.
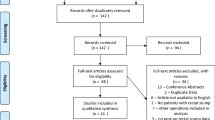
Following completion of the literature search, the studies were exported to the Rayyan software (Rayyan Systems Inc., Qatar) [12]. Duplicate studies were removed and studies were screened in a three-stage process; first by title, then by abstract and finally by full text. Studies were screened by two independent researchers (I.G. and S.M.) and any conflict was resolved by a third reviewer (D.B.). A PRISMA flowchart of the study screening process is displayed in Fig. 1 [10].
Inclusion criteria
(1) Peer reviewed published manuscripts that reported information on the incidence rates, surveillance techniques or risk factors for malignancy post IBD colectomy. (2) Papers describing the operation as total abdominal colectomy, total colectomy or subtotal colectomy were included due to the variation in definitions. (3) Retrospective, observational and population-based cohort studies and patient series were all included due to the generally low numbers of publications in this field. (4) Only studies published in English language and with at least 20 participants.
Exclusion criteria
(1) Studies including patients without a confirmed diagnosis of IBD. (2) patients with diagnoses of syndromes related to CRC such as familial adenomatous polyposis (FAP) or Lynch syndrome. (3) Colectomy procedures undertaken for diagnoses other than IBD. (4) Conference abstracts and studies (all small single-centre cohort studies, the largest of which had 42 patients) that were not available in the English language were excluded.
Primary and secondary outcomes
The primary outcomes of this systematic review were to estimate the published prevalence and incidence rates of malignancy in the residual rectum. The secondary outcome included identification of cohorts of patients at higher risk of rectal malignancy. Additionally, we reviewed any screening regimens from the available literature.
Data extraction
The data points relevant for analysis were agreed by the members of research group and each individual paper was explored to extract the relevant data. Data were stored and analysed on a Microsoft Excel spreadsheet (Microsoft, USA).
Critical appraisal
The studies were evaluated according to the Critical Appraisal Skills Programme criteria for cohort studies Checklist (CASP) 2018 [13] by two independent researchers (G.I. and M.S.). The criteria were used to examine for sources of bias, to evaluate the internal validity and to assess the reliability of the evidence. The results were recorded on a table using Word (Microsoft, USA).
Statistical analysis
Where appropriate, the statistical analysis was conducted in Review Manager (RevMan) V5.4.1 (the Cochrane Collaboration, UK) [14]. Pooled analysis, prevalence and median values were calculated using Excel (Miscrosoft, USA). For the outcome of risk stratification, a dichotomous analysis was undertaken by calculating the risk ratio (RR) with 95% confidence interval (CI). Heterogeneity between the studies was examined by I2 statistics [15]. A sensitivity analysis was performed for outcomes with significant heterogeneity.
Study registration
The study was registered in the Research Registry (reviewregistry1370).
Results
Study characteristics
In total, 1049 papers were screened and 24 studies were eligible for inclusion (Fig. 1) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39, 46]. The earliest paper included was published in 1977 and the most recent in 2021. There were 22 retrospective and two prospective studies. Of the included studies, three were population based and the remaining 21 were patient series. A total of four studies were multicentre, while the remaining 20 were single centre. Fourteen studies were undertaken in Europe (including seven in the UK), three in the USA, two in Asia and one in Australia. The follow-up ranged from 1.9 to 40 years. The study characteristics are described in Table 2.
Participant characteristics
A total of 12,666 patients were included across the 24 studies. The number of participants in each study ranged from 20 to 5470. There were 11,030 patients diagnosed with ulcerative colitis, 1613 with Crohn’s disease and 23 with IBD indeterminate colitis. Table 3 provides a summary of the population, intervention, control and outcome of each study.
Rate of rectal remnant malignancy
Data on the occurrence of rectal remnant malignancy was available from 23 papers. One study (Ehsanullah [33] had an overlapping population with Baker [37]. Therefore, Ehsanullah was only included in the surveillance outcome.The mean duration of follow-up varied between 2 and 20 years. The mean age at the time of the surgery was 35.6 years. The pooled incidence of rectal malignancy post IBD colectomy was 1.3% from a total of 12,424 patients (median: 1.9%; range: 0.0–10.0%). Table 4 lists the rates of rectal cancer in these patients, by publication.
The papers were published across a 44-year time frame. The differences in rates of malignancy across the time frame were investigated by calculating the malignancy rate for each paper published in chronological order. In doing this, we noted the lowest rates of malignancies were reported in the studies between 2011 and 2021. Supplementary Table 2 summarises the rates of malignancy by decade.
Rate of rectal malignancy in the rectal remnant in patients with ulcerative colitis (UC) and Crohn’s disease (CD)
A subgroup analysis was performed in studies that separated the subtypes of IBD into UC and CD (15 studies). Rectal malignancy in patients with UC was available from 13 studies. A total of 6881 patients were included, of whom 108 developed a rectal carcinoma. The pooled rate was 1.6% across the studies (range: 0.0–10.0%; median: 4.8%). Supplementary Table 3 summarises the rates of malignancy. A further subgroup analysis showed a pooled rate of 3.2% in 2503 UC patients with IRA available from 12 studies, and 0.6% in 4360 patients with a rectal stump reported in 2 studies. It refers to subgroup analysis examining Rectal Stump patients with UC which only involves Abdalla and Munie. Unfortunately the rest of the studies with Rectal Stump patients could not be included within this subgroup analysis (eg Hove, Porter) as they included patients from both subgroups (UC, CD), without specifying in which of these subgroups the patients with malignancy belonged to. Upon reviewing, the total number of patients is 4378 from 4360 (4358 Abdalla + 20 Munie).
Studies not reporting if the patients with cancer occurrence belonged in the UC or CD subgroup, were not included in this analysis.
The remaining two studies examining CD patients included 120 patients, and therefore not deemed sufficient for a pooled analysis
Prevalence of rectal stump and ileorectal anastomosis malignancy
A subgroup analysis was also performed on the malignancy rates within a de-functioned rectal stump (Supplementary Table 4) and in those with an IRA (Supplementary Table 5). Patients who had a de-functioned rectal stump were identified in seven papers (a total of 9444 patients). The pooled diagnosis rate was 0.7% (median: 1.4%; range: 0.0–10.0%,). A cumulative malignancy incidence of patients with a rectal stump was reported in two studies [16, 18] including 9061 patients. The weighted combined incidence was 0.3% at 10 years post surgery.
IRA patients were assessed in 17 papers with a total of 2980 patients. The pooled prevalence was 3.3% (range: 0.0–10.0%; median 3.4%). A cumulative malignancy incidence of patients with IRA was reported in four studies [18, 20, 21, 25] including 1571 patients. The weighted combined incidence was found to be 2% at 10 years, and 6.8% at 20 years post surgery.
Pooled incidence of malignancy in the rectal remnant
Pooled incidence was calculated with data from 16 studies [16,17,18,19,20,21,22,23,24,25, 29,30,31, 34, 35, 38] and 11,594 participants. Eleven studies [20,21,22, 24, 25, 29,30,31, 34, 35, 38] involved participants with IRA, four studies involved patients with a rectal stump [16, 17, 19, 23]and one included both [18]. The analysis showed that there were 6.5 cases per 100,000 patient-years (Supplementary Table 6).
Surveillance regimen
Information regarding surveillance was reported in 10 out of the 24 eligible studies. The year of publication of the nine studies ranged from 1985 to 2021. The studies were also geographically varied: three were done in the USA [28, 29, 32], seven in the UK [19, 27, 31, 36,37,38], two in Japan [21, 23] and three in northern Europe (Sweden [18], the Netherlands [17] and Denmark [16]). Endoscopic investigation was used across all the studies, and seven studies advocated performing biopsies for histological examination [22, 24, 25, 28, 29, 33, 36]. The use of dye spray to identify suspicious lesions in flat mucosa was also reported in one study [24]. In four of the studies, there was no reported data on the frequency of the examination [16, 22, 24, 36]. In two of the publications, the endoscopies were performed annually [21, 25], and in a further two papers, surveillance was between 3 months and 1 year [28, 29].
Finally, one study reported that malignancy was found on magnetic resonance imaging ( MRI), but it is not clear whether the scan was part of the surveillance protocol or if it was performed with a different intention [17]. In the remaining 13 studies, there is no information reported on any surveillance regimen that the population adhered to. Andersson et al. reported that patients underwent surveillance only when they were symptomatic or with a duration of the disease over 10 years [22]. They advise that patient characteristics and risk stratification need to be considered to provide an ideal and personalised screening plan for every individual [22].
No study referenced the use of specific guidelines to optimise the surveillance regimen. One study highlighted significant variability between the type and the interval of screening due to the lack of guidelines, emphasising the importance of standardised guidance [17]. Table 5 provides all the information provided regarding the surveillance regimens followed, by publication.
Risk stratification of malignancy in the rectal remnant
History of CRC
A total of five studies, published between 1978 and 2017, that evaluated a history of colorectal cancer in the colectomy resection as a risk factor for developing malignancy in the residual rectum were included [18, 20, 22, 32, 37]. The number of participants in these studies ranged from 105 to 5470. A total of 6433 patients were examined, of whom 276 had a history of CRC (Fig. 2). Thirteen out of the 276 patients were diagnosed with cancer in the residual rectum (pooled prevalence of 4.7%, Supplementary Table 7). On meta-analysis, CRC patients had a significantly higher risk of synchronous pathology in their rectum than patients without malignancy (RR 7.20, 95% CI 2.46–21.12, I2 65%, p = 0.0003, Fig. 2).
History of dysplasia
Data on a history of dysplasia within the colon was available in five studies published between 1981 and 2021[16, 18, 20, 34, 39]. The number of participants in these studies was between 50 and 5470. They included a total of 10,700 patients, of whom 165 had a history of a biopsy showing dysplasia and 10,485 had no history of dysplasia. Twenty patients out of the 132 with a history of dysplasia were diagnosed with rectal malignancy. Patients with dysplasia were more likely to develop malignancy in a residual rectum compared with patients without a history of dysplasia (RR 5.07, 95% CI 3.11–8.24, I2 0%, p < 0.0001, Fig. 3).
Heterogeneity and sensitivity analysis
Heterogeneity was found to be significant (I2 65%) on meta-analysis exploring history of colorectal cancer. A sensitivity analysis was performed by removing studies one by one and assessing the effect. One study [18] contributed the majority of the heterogeneity, possibly a result of being a larger cohort than the rest of the included publications. Excluding this study resulted in an increased risk ratio. However, this study was one of the larger studies as it was a multicentre cohort and patients were recruited from a registered database. The study could therefore not be justifiably excluded from the analysis.
Critical appraisal
The critical appraisal showed that the majority of studies were of low quality. There were no large datasets and most were case series. However, 15 of the 24 studies met at least 10 out of the 11 criteria [16,17,18, 20,21,22,23, 25, 27, 28, 30, 33,34,35, 37]. Eight studies were positive for 7–9 out of 11 criteria [19, 24, 26, 29, 31, 36, 39, 46], while one of the studies met only six of the criteria [32]. The summary of quality assessment for the 24 included studies is detailed in Supplementary Table 8.
Discussion
In this systematic review and meta-analysis of the incidence, risk factor stratification and surveillance strategy for rectal malignancy in post total colectomy IBD patients, we have identified some key findings. Firstly, the pooled prevalence of residual rectum malignancy after colectomy across the literature is 1.3%. Interestingly, this is lower than that quoted previously, which has been 3% [9]. Such a finding is key for patient counselling in terms of assessment of the rectum after colectomy, surveillance stratification and decisions regarding further management.
Given that medical management aims to reduce inflammation and that a pro-inflammatory state potentiates malignancy [40], it may be that the lower rates reflect the long-term effects of immuno-biologic medications introduced in the early 2000s. However, it is likely to be many years before we will be able to confirm this hypothesis. It is, nevertheless, intriguing that, in this assessment of rates of malignancy across the 44 years of the publications available, the decade with the lowest rate of cancer detection was the most recent.
A further key finding of this study is that there is standardised screening guidance for this group. We identified a common trend in the reported frequency of surveillance: endoscopic examination performed annually or biannually. The absence of any guidance means that surveillance is currently at the discretion of the clinician, making service provision challenging.
Adhering to screening guidelines that are designed for patients with an intact bowel can result in exposing patients to unnecessary tests that could potentially cause harm and discomfort [41], and such approaches may not be cost effective. There is a further question of accuracy of surveillance. Luminal investigations may be challenging if the rectal stump has been strictured down, preventing adequate visualisation of the upper aspect of the rectal remnant. MRI of the pelvis may be helpful in this setting [42]. However, there are insufficient data from the papers identified in this review to comment further on surveillance.
The pooled prevalence of malignancy in the rectal stump and IRA in this review was 0.7% and 3.2% respectively. Previous literature has reported rates of 2.1% for patients with a de-functioned rectum and 2.4% for IRA patients [9]. Overall, our findings indicate the malignancy risk in these cohorts is still lower than the general population lifetime risk of developing CRC, which is estimated at 4.4% [43]. Given the inherent differences in the rectal stump and IRA patient cohorts it is not possible to comment further on whether this difference in malignancy detection is anything other than differences in the patient cohorts.
A history of CRC was found to be a risk factor, which agrees with existing literature for both the general population with IBD and for IBD patients with total colectomy [9, 44]. An interesting finding of this study is that the pooled prevalence of cancer recurrence after a colorectal primary was 4.7%. In contrast, a recent study published in 2016 reported that 17% of the participants who were treated for CRC with a curative-intent experienced recurrence [45].
It is important to acknowledge that surgical and endoscopic techniques have changed over time, with ileal pouch–anal anastomosis a common surgical procedure which necessitates rectal resection. Consequently, rectal cancer risk is reduced. However, such a procedure is not without risk. Adverse events such as effects on female fecundity and pelvic nerve damage must be taken into account when counselling patients for such procedures [46, 47].
One of the limitations of this study is the large number of low-quality studies and the inclusion of only few large patient cohorts. Larger data could be retrieved from a national registry of IBD management. However, to date, no such registry exists. [48]. Another limitation is that only English language studies were included. However, non-English studies identified on abstract review that could potentially have been eligible were small cohort studies which were unlikely to influence the results.
Conclusions
The pooled analysis of rectal cancer was reported at 1.3% for IBD patients with both an IRA and a rectal stump. History of colorectal cancer and dysplasia was associated with developing malignancy in the residual rectum. However, this is an understudied area with few large-scale good-quality studies. Furthermore, no consistent guidance for surveillance of this group currently exists.
Data availability
The data that support the findings of this study are openly available in the reference list provided below.
References
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF (2017) Ulcerative colitis. Lancet 389:1756–1770. https://doi.org/10.1016/S0140-6736(16)32126-2
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657. https://doi.org/10.1016/S0140-6736(07)60751-X. (PMID: 17499606)
Ullman TA, Itzkowitz SH (2011) Intestinal inflammation and cancer. Gastroenterology 140:1807–1816. https://doi.org/10.1053/j.gastro.2011.01.057. (PMID: 21530747)
Axelrad J E et al (2016) Inflammatory bowel disease and cancer: the role of inflammation immunosuppression, and cancer treatment. World J Gastroenterol. https://doi.org/10.3748/wjg.v22.i20.4794
Pithadia AB, Jain S (2011) Treatment of inflammatory bowel disease (IBD). Pharmacol Rep 63:629–642. https://doi.org/10.1016/s1734-1140(11)70575-8
Hwang JM, Varma MG (2008) Surgery for inflammatory bowel disease. World J Gastroenterol 14:2678–2690. https://doi.org/10.3748/wjg.14.2678
Lamb CA, Kennedy NA, Raine T et al (2019) British society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. https://doi.org/10.1136/gutjnl-2019-318484
Brown SR, Fearnhead NS, Faiz OD (2018) The association of coloproctology of Great Britain and Ireland consensus guidelines in surgery for inflammatory bowel disease. Colorectal Dis. https://doi.org/10.1111/codi.14448
Derikx LAAP, Nissen LHC, Smits LJT, Shen B, Hoentjen F (2016) Risk of neoplasia after colectomy in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 14:798–806. https://doi.org/10.1016/j.cgh.2015.08.042
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Inter J Surgery. https://doi.org/10.1186/s13643-021-01626-4
Shea BJ, Reeves BC, Wells G, et al. (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ Sep 21 358 j4008
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Critical Appraisal Skills Programme (2022). CASP (cohort study) Checklist https://casp-uk.net/casp-tools-checklists/
Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020.
J.P.T. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, (2003) Measuring inconsistency in meta-analyses BMJ https://doi.org/10.1136/bmj.327.7414.557. PMID: 12958120; PMCID: PMC192859.
Mark-Christensen A, Erichsen R, Veres K, Laurberg S, Sørensen HT (2021) Rectal cancer risk and survival after total colectomy for IBD: a population-based study. Dis Colon Rectum 64:583–591. https://doi.org/10.1097/DCR.0000000000001810
Ten Hove JR, Bogaerts JMK, Bak MTJ, Laclé MM, Meij V, Derikx LAAP, Hoentjen F, Mahmmod N, van Tuyl SA, Oldenburg B (2019) Malignant and nonmalignant complications of the rectal stump in patients with inflammatory bowel disease. Inflamm Bowel Dis 25:377–384. https://doi.org/10.4240/wjgs.v13.i2.198
Abdalla M, Landerholm K, Andersson P, Andersson RE, Myrelid P (2017) Risk of rectal cancer after colectomy for patients with ulcerative colitis: a national cohort study. Clin Gastroenterol Hepatol 15:1055–1060. https://doi.org/10.1016/j.cgh.2016.11.036
Porter DJ, Lucocq J, Muthukumarasamy G (2021) The fate of the rectal stump following subtotal colectomy for acute colitis. World J Surg Surgical Res 4:1321
Uzzan M, Kirchgesner J, Oubaya N et al (2017) Risk of rectal neoplasia after colectomy and ileorectal anastomosis for ulcerative colitis. J Crohns Colitis 11:930–935. https://doi.org/10.1093/ecco-jcc/jjx027
Ishii H, Hata K, Kishikawa J, Anzai H et al (2016) Incidence of neoplasias and effectiveness of postoperative surveillance endoscopy for patients with ulcerative colitis: comparison of ileorectal anastomosis and ileal pouch-anal anastomosis. World J Surg Oncol 14:75. https://doi.org/10.1186/s12957-016-0833-5
Andersson P et al (2014) Ileorectal anastomosis in comparison with ileal pouch anal anastomosis in reconstructive surgery for ulcerative colitis - a single institution experience. J Crohn’s Colitis. https://doi.org/10.1016/j.crohns.2013.11.014
Munie S, Hyman N, Osler T (2013) Fate of the rectal stump after subtotal colectomy for ulcerative colitis in the era of ileal pouch-anal anastomosis. JAMA Surg 148:408–411. https://doi.org/10.1001/jamasurg.2013.177
Shuno Y, Hata K, Sunami E, Shinozaki M, Kawai K, Kojima T, Tsurita G et al (2011) 2011 Is surveillance endoscopy necessary after colectomy in ulcerative colitis? ISRN Gastroenterol. https://doi.org/10.5402/2011/509251
da Luz MA, Kiran RP, Lavery I (2010) Clinical outcomes of ileorectal anastomosis for ulcerative colitis. Br J Surg 97:65–69. https://doi.org/10.1002/bjs.6809
Winther KV, Bruun E, Federspiel B, Guldberg P, Binder V, Brynskov J (2004) Screening for dysplasia and TP53 mutations in closed rectal stumps of patients with ulcerative colitis or crohn disease. Scand J Gastroenterol 39:232–237. https://doi.org/10.1080/00365520310008368
Yamamoto T, Keighley MRB (1999) Long-term outcome of total colectomy and ileostomy for crohn disease. Scand J Gastroenterol 34:280–286. https://doi.org/10.1080/00365529950173690
Pastore RL, Wolff BG, Hodge D (1997) Total abdominal colectomy and ileorectal anastomosis for inflammatory bowel disease. Dis Colon Rectum 40:1455–1464. https://doi.org/10.1007/BF02070712
Khubchandani IT, Kontostolis SB (1994) Outcome of ileorectal anastomosis in an inflammatory bowel disease surgery experience of three decades. Arch Surg 129:866–869. https://doi.org/10.1001/archsurg.1994.01420320092018
Leijonmarck CE, Löfberg R, Ost A, Hellers G (1990) Long-term results of ileorectal anastomosis in ulcerative colitis in stockholm county. Dis Colon Rectum 33:195–200. https://doi.org/10.1007/BF02134178
Thomas DM, Filipe MI, Smedley FH (1989) Dysplasia and carcinoma in the rectal stump of total colitics who have undergone colectomy and ileo-rectal anastomosis. Histopathology 14:289–298. https://doi.org/10.1111/j.1365-2559.1989.tb02147.x
Oakley G, O’Dwyer ST (2006) The fate of the rectal stump after subtotal colectomy for ulcerative colitis. Inter J Colorectal Disease, Springer Link 22:277–282. https://doi.org/10.1007/s00384-006-0127-4
Ehsanullah M, Naunton Morgan M, Filipe MI, Gazzard B (1985) Sialomucins in the assessment of dysplasia and cancer-risk patients with ulcerative colitis treated with colectomy and ileo-rectal anastomosis. Histopathology 9:223–235. https://doi.org/10.1111/j.1365-2559.1985.tb02437.x
Grundfest SF, Fazio V, Weiss RA, Jagelman D, Lavery I, Weakley FL, Turnbull RB Jr (1981) The risk of cancer following colectomy and ileorectal anastomosis for extensive mucosal ulcerative colitis. Ann Surg 193:9–14. https://doi.org/10.1097/00000658-198101000-00002
Farnell MB, Van Heerden JA, Beart RW Jr, Weiland LH (1980) Rectal preservation in nonspecific inflammatory disease of the colon. Ann Surg 192:249–253
Jones PF, Bevan PG, Hawley PR (1978) Ileostomy or ileorectal anastomosis for ulcerative colitis? Br Med J 1:1459–1463. https://doi.org/10.1097/00000658-198008000-00021
Baker WN, Glass RE, Ritchie JK, Aylett SO (1978) Cancer of the rectum following colectomy and ileorectal anastomosis for ulcerative colitis. Br J Surg 65:862–868. https://doi.org/10.1002/bjs.1800651211
Watts JM, Hughes ES (1977) Ulcerative colitis and Crohn’s disease: results after colectomy and ileorectal anastomosis. Br J Surg 64:77–83. https://doi.org/10.1002/bjs.1800640202
Johnson WR, McDermott FT, Pihl E, Hughes ES (1983) Mucosal dysplasia a major predictor of cancer following ileorectal anastomosis. Dis Colon Rectum. 26(11):697–700
Colombel JF, D’haens G, Lee WJ, Petersson J, Panaccione R (2020) Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. https://doi.org/10.1093/ecco-jcc/jjz131
Haas PA (1969) Complications of proctoscopy and irrigoscopy. Progress in Proctology, Springer. https://doi.org/10.1007/978-3-642-87959-3_3
Delli Pizzi A, Basilico R, Cianci R et al (2018) Rectal cancer MRI: protocols, signs and future perspectives radiologists should consider in everyday clinical practice. Insights Imaging. https://doi.org/10.1007/s13244-018-0606-5
Siegel, Rebecca L., et al, (2020) Cancer Statistics American, Cancer Society Journals, American Cancer Society https://doi.org/10.3322/caac.21590.
Das, Ananya M.D.1; Chak, Amitabh M.D.2; Cooper, Gregory S. (2006) MD2, Temporal trend in relative risk of second primary colorectal cancer. Am J Gastroenterology https://doi.org/10.1111/j.1572-0241.2006.00580.x.
Duineveld LA, van Asselt KM, Bemelman WA, Smits AB, Tanis PJ, van Weert HC, Wind J (2016) Symptomatic and asymptomatic colon cancer recurrence: a multicenter cohort study. Ann Fam Med 14:215–220. https://doi.org/10.1370/afm.1919
Johnson P, Richard C, Ravid A et al (2004) Female infertility after ileal pouch–anal anastomosis for ulcerative colitis. Dis Colon Rectum 47:1119–1126. https://doi.org/10.1007/s10350-004-0570-7
Gorgun E, Remzi FH, Goldberg JM et al (2004) Fertility is reduced after restorative proctocolectomy with ileal pouch anal anastomosis: a study of 300 patients. Surgery 136:795–803. https://doi.org/10.1016/j.surg.2004.06.018
Brown SR et al (2018) ACPGBI IBD surgery consensus collaboration, the association of coloproctology of great Britain and Ireland consensus guidelines in surgery for inflammatory bowel disease. Colorectal Dis 20(Suppl 8):3–117. https://doi.org/10.1111/codi.14448
Acknowledgements
The authors would like to kindly thank Mr. Rob Polson for his valuable assistance with the search strategy.
Funding
There was no funding provided for this study.
Author information
Authors and Affiliations
Contributions
Conception and design: A.W., G.R., C.P., S.M. and IG. Search strategy development: I.G. and S.M. Record screening and critical appraisal: I.G., S.M. and D.B. Data extraction: I.G. and S.M. Data analysis: I.G., S.M. and G.R. Writing: A.W., I.G., P.C., R.G. and D.B. Critical revision of the article: A.W., G.R. and C.P.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As this was a review of previously published literature, we did not seek further ethical review as we felt this was not required.
Research registration
Research registry, unique identifying number: reviewregistry1370.
Informed Consent
For this type of study, informed consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Georganta, I., McIntosh, S., Boldovjakova, D. et al. The incidence of malignancy in the residual rectum of IBD patients after colectomy: a systematic review and meta-analysis. Tech Coloproctol 27, 699–712 (2023). https://doi.org/10.1007/s10151-023-02762-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-023-02762-w