Abstract
Background
Postoperative ileus (POI) is a common complication following colorectal surgery and is mediated in part by the cholinergic anti-inflammatory pathway (CAIP). Neostigmine (acetylcholinesterase inhibitor), co-administered with glycopyrrolate, is frequently given for neuromuscular reversal before tracheal extubation and modulates the CAIP. An alternative reversal agent, sugammadex (selective rocuronium or vecuronium binder), acts independently from the CAIP. The aim of our study was to assess the impact of neuromuscular reversal agents used during anaesthesia on gastrointestinal recovery.
Methods
Three hundred thirty-five patients undergoing elective colorectal surgery at the Royal Adelaide Hospital between January 2019 and December 2021 were retrospectively included. The primary outcome was GI-2, a validated composite measure of time to diet tolerance and passage of stool. Demographics, 30-day complications and length of stay were collected. Univariate and multivariate analyses were performed.
Results
Two hundred twenty-four (66.9%) patients (129 [57.6%] males and 95 [42.4%] females, median age 64 [19–90] years) received neostigmine/glycopyrrolate and 111 (33.1%) received sugammadex (62 [55.9%] males and 49 [44.1%] females, median age 67 [18–94] years). Sugammadex patients achieved GI-2 sooner after surgery (median 3 (0–10) vs. 3 (0–12) days, p = 0.036), and reduced time to first stool (median 2 (0–10) vs. 3 (0–12) days, p = 0.035). Rates of POI, complications and length of stay were similar. On univariate analysis, POI was associated with smoking history, previous abdominal surgery, colostomy formation, increased opioid use and postoperative hypokalaemia (p < 0.05). POI was associated with increased complications, including anastomotic leak and prolonged hospital stay (p < 0.001). On multivariate analysis, neostigmine, bowel anastomoses and increased postoperative opioid use (p < 0.05) remained predictive of time to GI-2.
Conclusions
Patients who received sugammadex had a reduced time to achieving first stool and GI-2. Neostigmine use, bowel anastomoses and postoperative opioid use were associated with delayed time to achieving GI-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative ileus (POI) is a common complication following major abdominal surgery, particularly colorectal surgery, occurring in up to 25% of patients resulting in significant morbidity and mortality [1]. POI occurs in two phases: an initial neurogenic phase followed by a secondary inflammatory phase [1]. The inflammatory phase starts approximately 3 h postoperatively, releasing inflammatory mediators that affect bowel function for a variable length of time [1, 2]. This inflammatory cascade is mediated, in part, by the cholinergic anti-inflammatory pathway (CAIP) [3, 4].
To facilitate abdominal surgery, most patients are paralysed with a non-depolarising neuromuscular blocking drug (NMBD) on induction. These agents competitively antagonise acetylcholine at postsynaptic nicotinic receptors in the neuromuscular junction (NMJ) [5]. Upon completion of surgery, any residual paralysis is reversed before tracheal extubation of the patient with either acetylcholinesterase inhibitors, most commonly neostigmine, or an encapsulating agent named sugammadex. Acetylcholinesterase inhibitors competitively bond with acetylcholinesterase in the synaptic cleft of the NMJ, reducing the hydrolysis of acetylcholine [6]. The increased concentration of acetylcholine competitively reverses the action of the NMBD at the NMJ [7]. The increase in acetylcholine, however, is not limited to the NMJ [8]. Peripheral muscarinic receptors also use acetylcholine and, if left unopposed, produce muscarinic side effects thus require co-administration of an anticholinergic agent (such as glycopyrrolate). The effect of neostigmine and glycopyrrolate as neuromuscular reversal agents on the CAIP and their overall impact on bowel motility following surgery remains unclear [9].
Sugammadex is a modified γ-cyclodextrin that encapsulates the aminosteroid NMBDs, rocuronium and vecuronium, with high affinity [10]. Sugammadex is a large molecule that does not readily enter the NMJ; acting mainly within the circulating plasma. Free NMBD molecules in the plasma are rapidly chelated, creating a concentration gradient promoting the movement of NMBD from the NMJ into the plasma where they are once again sequestered [8]. The reduction in NMBD available at the NMJ, results in the reversal of the neuromuscular blockade. Sugammadex acts independently of cholinergic transmission and therefore does not require co-administration of anticholinergic agents, and thus has no potential to act on the CAIP [11]. Sugammadex is, however, speculated to alter gut motility and gastric emptying due to its affinity to bind with steroid hormones [12, 13].
As sugammadex and neostigmine could influence the return of bowel function, several studies have investigated their impact with varied results [12,13,14,15,16]. However, these studies do not compare neostigmine and sugammadex using a validated gastrointestinal recovery outcome measure, such as GI-2 [17]. Our aim was to identify the effect of neostigmine/glycopyrrolate or sugammadex on gastrointestinal recovery following colorectal surgery using GI-2.
Materials and methods
This study is reported using the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines [18], and was approved by the Central Adelaide Local Health Network Human Research Ethics Committee. A waiver of consent for retrospective patients was provided in accordance with the guidelines provided by the National Health and Medical Research Council’s (NHMRC) [19].
Patient selection
This study was performed at the Colorectal Unit of the Royal Adelaide Hospital (RAH), a tertiary referral centre in South Australia, Australia. Patients were identified from the elective admission lists and underwent surgery between January 2019 and December 2021. All patients at the RAH, are placed on an enhanced recovery pathway (ERP). The ERP protocol can be found at www.tinyurl.com/raheras.
Inclusion and exclusion criteria
Consecutive elective colorectal patients over 18 years old who underwent major bowel surgery, consisting of large or small bowel resection, reversal or stoma formation, were included. Pelvic exenterations were excluded due to the associated high morbidity and variables affecting return of bowel function. Robotic cases were excluded as they are performed at another geographic site and transferred to the study hospital for postoperative care. Patients who did not receive a neuromuscular reversal agent, received both agents, non-operative admissions, or prescribed acetylcholinesterase inhibitors as part of the ‘Pyridostigmine to reduce the incidence of postoperative ileus following colorectal surgery (PyRICo – P)’ study were excluded [20].
Data collection
Data were collected retrospectively from paper and electronic medical records by two authors (LT and TH). Anaesthetist choice of neostigmine/glycopyrrolate or sugammadex was collected. Known risk factors for the development of POI were collected [21,22,23]. Baseline demographics such as age, body mass index (BMI), smoking history, congestive cardiac failure (CCF), chronic obstructive pulmonary disease (COPD), hypertension, diabetes mellitus, regular steroid use, ascites or previous abdominal surgery history were recorded, along with preoperative haemoglobin, total protein and albumin. Operative data included the diagnosis (benign/malignant), surgical approach (open/laparoscopic), laparoscopic to open conversion, procedure type, stoma formation and duration of surgery, and intraoperative and postoperative fluid administration. Postoperative data included opioid requirements in morphine equivalents (intraoperative, postoperative recovery and day one to four use) calculated using Opioid Calculator v2.9.1 (Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists, Australia),
Outcomes
The primary outcome was gastrointestinal recovery measured retrospectively using GI-2: a validated outcome measure comprised of time to first stool and tolerance of solid diet without significant nausea or vomiting [17]. Secondary outcomes included POI, defined as not achieving GI-2 by day 4 postoperatively, as well as time to first stool, time to tolerance of oral diet, and nasogastric tube (NGT) reinsertion incidence for both groups. Furthermore, postoperative outcomes including intensive care admission and length of stay were recorded. Thirty-day complications, Clavien-Dindo (CD) grades, return to theatre, and readmission rates were collected [24]. Anastomotic leak was defined by patients having extra-luminal presence of contrast fluid on a contrast-enhanced computed tomography scan and/or evidence of leakage of luminal contents from a surgical join on reintervention within 30 days [25].
Statistical analysis
A priori power calculation was performed using G*Power 3.1 (Franz Faul, Universitat Kiel, Germany), with the best available data from Hunt et al. showing a mean return of stool with sugammadex of 1.7d (SD 1.2) and 2.2d (SD 1.3) (converted from hours) with neostigmine, as no previous studies used GI-2 [16]. Using an α error of 0.05, ß error of 0.2, power of 0.8 and an effect size of 0.40, a minimum sample size of 100 patients in each arm was required. Numerical data are presented as median (IQR [range]) or mean (standard deviation) depending on parametricity identified with the Shapiro–Wilk test. Univariate analysis was performed using the Mann–Whitney U for nonparametric variables or student's t test for normally distributed continuous variables. The χ2 or Fisher’s exact test (when expected n < 5) for categorical variables. All collected variables were used in the univariate linear regression analysis on log-normal transformed time to GI-2. Statistically significant variables were then used for multivariate linear regression analyses, to determine predictors of GI-2. Data for multivariate linear regression analyses were evaluated and met all linear assumptions. P values of < 0.05 were considered statistically significant. A 1-day reduction in GI-2 was considered clinically significant. Statistical analysis was performed using SPSS 28.0 (SPSS Inc., Armonk, NY, USA).
Results
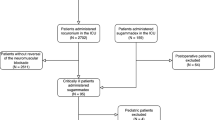
Of 1115 elective colorectal admissions during the study period, 335 patients were included (Fig. 1). 224 (66.9%) patients received neostigmine and glycopyrrolate (129 [57.6%] males and 95 [42.4%] females, median age 64 [19–90] years), and 111 (33.1%) received sugammadex (62 [55.9%] males and 49 [44.1%] females, median age 67 [18–94] years). Three patients in the neostigmine group were also given atropine, and seven patients in the sugammadex received glycopyrrolate to treat intraoperative bradycardia. Both groups’ baseline patient and operative characteristics are summarised in Table 1. Patients receiving sugammadex had a higher ASA class > 3 (60.4 vs. 45.1%, p < 0.001), a greater BMI (median 28.7 vs. 26.8 kg/m2, p = 0.003), were more comorbid with COPD (15.3 vs. 6.7%, p = 0.012) and hypertension (56.8 vs. 41.5%, p = 0.008) and were more likely to undergo laparoscopic surgery (66.7 vs. 50.9%, p = 0.006).
Postoperatively, patients receiving sugammadex had a statistically significantly shortened median time to GI-2 (3 (0–10) vs. 3 (0–12) days, p = 0.036) and a reduced median time to first stool (2 (0–10) vs. 3 (0–12), p = 0.035) (Table 2). There were no significant differences in time to POI rates, NGT reinsertion, length of stay and 30-day complications between groups (Table 2).
Overall, 93 patients (27.8%) had a POI (Table 3). POI was more likely to occur in patients with a history of smoking (62.3 vs. 45.9%, p = 0.025), previous abdominal surgery (68.8 vs. 53.7%, p = 0.012), those who underwent open surgery (55.9 vs. 39.3%, p = 0.006), and patients who had a colostomy formed (60.0 vs. 22.8%, p = 0.005). Patients within postoperative day 1–4 with lower potassium (median 3.7 vs. 3.8 mmol/L, p = 0.017), charted aperients (69.9 vs. 55.4%, p = 0.015) and receiving more postoperative opioids (median 218 vs. 110 MEQ, p < 0.001) developed POI. POI was associated with significantly more ICU admissions (9.7 vs. 2.1%, p = 0.002), anastomotic leaks (13.9 vs. 2.3%, p < 0.001), greater incidence of return to theatre (8.6 vs. 2.5%, p = 0.012) and a higher CD grade of complications (p < 0.001). Patients diagnosed with a POI had a 3-day increase in median length of stay (8 (3–33) vs. 5 (1–60) days, p < 0.001).
On univariate and multivariate linear regression analyses, neostigmine/glycopyrrolate use (p = 0.034), anastomosis formation (p < 0.001) and increased postoperative opioid use were predictive of time to achieving GI-2 (p < 0.001) (Table 4).
Discussion
This study demonstrates a statistically but not clinically relevant difference in time to GI-2 achievement favouring sugammadex used in neuromuscular reversal compared to neostigmine. We also found a clinically significant 1-day reduction in time to first stool favouring sugammadex use. However, the choice of neuromuscular reversal agent did not impact the incidence of POI as defined by GI-2.
These results support previous studies that have demonstrated a reduced time to return of gastrointestinal function with sugammadex. In abdominal surgery studies, sugammadex resulted in an earlier return of flatus when investigating laparoscopic cholecystectomy, but no change in time to first stool [13]. The most extensive study to date included over 8000 patients undergoing abdominal surgery without differentiating types of surgery. It investigated the impact of reversal agents on gastrointestinal recovery, showing that sugammadex resulted in a faster first bowel movement than neostigmine [14]. Several studies have also investigated colorectal surgical patients, favouring sugammadex [15, 16]. In our cohort, although sugammadex patients had an earlier time to first stool, there was no reduction in the risk of developing POI and no clinical difference in time taken for gastrointestinal recovery as defined by GI-2.
Neostigmine did not have a beneficial effect on the return of GI function postoperatively, and there are several plausible explanations for this. The overall duration of action for neostigmine is 20–30 min [26]. Given that the CAIP develops from approximately 3 h postoperatively, this could explain why there is little impact on POI rates. In addition, while historical evidence suggested that co-administration with glycopyrrolate would not reverse the promotility effect of neostigmine [27], contemporary studies have suggested this does lead to a delay in return of gastrointestinal recovery following intraperitoneal surgery [14]. The delay in the return of gastrointestinal function likely results from neostigmine’s cholinergic effects being negated due to its co-administration of the anticholinergic glycopyrrolate. This is supported by the pharmacology of glycopyrrolate, with the duration of action being three to five times longer than neostigmine [28]. This accounts for the observed outcomes of the current study compared to sugammadex, a selective agent without anticholinergic activity [29].
In our study, the reversal agent was chosen by anaesthetist preference, without surgical input. Patients receiving sugammadex were more overweight and comorbid. Compared to neostigmine, sugammadex demonstrates a faster onset of reversal, the potential to reverse deeper neuromuscular blockade, decreased postoperative nausea and vomiting, shortened recovery time, and minimal side effects [30]. Hence, sugammadex was chosen to reverse these higher risk patients to minimise postoperative morbidity. Despite this, the differences in comparing neostigmine/glycopyrrolate and sugammadex, such as BMI and comorbidities, were not identified on multivariate analysis to predict increased GI-2. We, therefore, postulate that these variables do not account for the differences in return of gastrointestinal function.
On multivariate linear regression analysis, bowel anastomoses formation, increased postoperative opioid use and neostigmine use were predictors for a prolonged time to achieving GI-2. Postoperative opioid use has clear associations with delayed return of gastrointestinal function, resulting in increased complications, length of hospital stay and hospital costs [22, 23]. Postoperative opioid use is a modifiable risk factor, with opioid avoidance strategies and interventions such as alvimopan, showing improvements in time to achieve GI-2 [31]. Other studies have also demonstrated, as in our cohort, a link between anastomosis formation and delayed return of bowel function, likely due to increased operative bowel handling [21, 32]. This is also supported by an open surgical approach being associated with delay in return of GI-2, although this did not reach significance on multivariate analyses.
For clinicians, the regular use of sugammadex over neostigmine/glycopyrrolate for neuromuscular reversal is hindered for a few key reasons. During the period of this study, the cost of sugammadex was AU$125 and neostigmine/glycopyrrolate was significantly cheaper at AU$3. The benefits of sugammadex outlined in previous studies and the current study do not outweigh the discrepancy in cost between the two medications [33]. A randomised-blinded study will be required to truly identify the impact sugammadex has on GI-2 and time to first stool. Should this demonstrate a significant clinical improvement in gastrointestinal function recovery, the regular use of sugammadex as part of an ERP could be economically justified, given the financial impact of POI [34]. Furthermore, sugammadex has the potential to cause anaphylaxis [33]. Although this is rare, neostigmine has no risk of anaphylaxis. Given the financial cost of sugammadex and the risk of anaphylaxis, the use of sugammadex for patients remains judicious.
This study had several limitations. This study was retrospective in design. Although there was an attempt to reduce bias using consecutive patients with strict inclusion and exclusion criteria, all selection biases cannot be eliminated. Also, some data points were missing. The baseline characteristics between sugammadex and neostigmine patients differed due to anaesthetist selection based on patient factors. Furthermore a propensity-matched analysis was unable to be performed, as the ratio of the number of relevant predictive variables to the total number of patients in the denominator was too high to present a meaningful analysis. To assess the effects of acetylcholinesterase inhibitors on the development of POI, we are currently recruiting for a double-blinded randomised controlled trial using postoperative acetylcholinesterase inhibitors (pyridostigmine) to investigate this question further (ACTRN:12621000530820).
Conclusions
This dataset forms the largest cohort of colorectal patients investigating the impact of neostigmine/glycopyrrolate and sugammadex use as neuromuscular reversal agents against the validated outcome of GI-2. Sugammadex use was associated with a shorter time to first stool and GI-2. However, the selection of neuromuscular reversal agents had no significant clinical impact on the development of POI. On multivariate analysis, neostigmine use, bowel anastomoses and increased postoperative opioid use were associated with delayed achievement of GI-2.
Data availability
Not applicable.
Code availability
Not applicable.
References
Boeckxstaens GE, De Jonge WJ (2009) Neuroimmune mechanisms in postoperative ileus. Gut 58(9):1300–1311. https://doi.org/10.1136/gut.2008.169250
Chapman SJ, Wells CI (2018) Challenges in ileus research. Colorectal Dis 20(7):639. https://doi.org/10.1111/codi.14239
Matteoli G, Gomez-Pinilla PJ, Nemethova A et al (2014) A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63(6):938–948. https://doi.org/10.1136/gutjnl-2013-304676
The FO, Boeckxstaens GE, Snoek SA et al (2007) Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology 133(4):1219–1228. https://doi.org/10.1053/j.gastro.2007.07.022
Murphy GD, de Boer HD, Eriksson LI, Miller RD (2020) Reversal (Antagonism) of Neuromuscular Blockade. In: Gropper MA (ed) Miller’s anesthesia, 9th edn. Elsevier, Philadelphia, pp 832–864
Hunter J, Shields M (2019) Muscle function and neuromuscular blockade. Smith and Aitkenhead’s Textbook of Anaesthesia, 7th edn. Elsevier, New York, pp 131–146
Neely Ga, Sabir S, Kohli A (2021) Neostigmine. In: StatPearls Publishing. Treasure Island (FL) https://www.ncbi.nlm.nih.gov/books/NBK470596/. Accessed 6 Jun 2021
Srivastava A, Hunter JM (2009) Reversal of neuromuscular block. Br J Anaesth 103(1):115–129. https://doi.org/10.1093/bja/aep093
Traeger L, Kroon HM, Bedrikovetski S, Moore JW, Sammour T (2021) The impact of acetylcholinesterase inhibitors on ileus and gut motility following abdominal surgery: a clinical review. ANZ J Surg 92(1–2):69–76. https://doi.org/10.1111/ans.17418
Schaller SJ, Fink H (2013) Sugammadex as a reversal agent for neuromuscular block: an evidence-based review. Core Evidence. https://doi.org/10.2147/CE.S35675
Caldwell JE, Miller RD (2009) Clinical implications of sugammadex. Anaesthesia 64:166–172. https://doi.org/10.1111/j.1365-2044.2008.05872.x
Sen A, Erdivanli B, Tomak Y, Pergel A (2016) Reversal of neuromuscular blockade with sugammadex or neostigmine/atropine: effect on postoperative gastrointestinal motility. J Clin Anesth 32(8):208–213. https://doi.org/10.1016/j.jclinane.2016.03.010
An J, Noh H, Kim E, Lee J, Woo K, Kim H (2020) Neuromuscular blockade reversal with sugammadex versus pyridostigmine/glycopyrrolate in laparoscopic cholecystectomy: a randomized trial of effects on postoperative gastrointestinal motility. Korean J Anesthesiol 73(2):137–144. https://doi.org/10.4097/kja.19360
Deljou A, Schroeder DR, Ballinger BA, Sprung J, Weingarten TN (2019) Effects of sugammadex on time of first postoperative bowel movement: a retrospective analysis. Mayo Clin Proc: Innov, Qual & Outcomes 3(3):294–301. https://doi.org/10.1016/j.mayocpiqo.2019.06.003
Chae YJ, Joe HB, Oh J, Lee E, Yi IK (2019) Thirty-day postoperative outcomes following sugammadex use in colorectal surgery patients: retrospective study. J Clin Med 8(1):97. https://doi.org/10.3390/jcm8010097
Hunt ME, Yates JR, Vega H, Heidel RE, Buehler JM (2020) Effects on postoperative gastrointestinal motility after neuromuscular blockade reversal with sugammadex versus neostigmine/Glycopyrrolate in colorectal surgery patients. Ann Pharmacother 54(12):1165–1174. https://doi.org/10.1177/1060028020929061
Van Bree SH, Bemelman WA, Hollmann MW et al (2014) Identification of clinical outcome measures for recovery of gastrointestinal motility in postoperative ileus. Ann Surg 259(4):708–714. https://doi.org/10.1097/SLA.0b013e318293ee55
Von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
National Health and Medical Research Council (NHMRC) Australian Research Council Australian Vice-Chancellors' Committee. (2018) National statement on ethical conduct in human research. Australia: NHMRC, Canberra. Report No.: ISBN: 1864962755.
Dudi-Venkata NN, Kroon HM, Bedrikovetski S et al (2021) PyRICo-Pilot: pyridostigmine to reduce the duration of postoperative ileus after colorectal surgery - a phase II study. Colorectal Dis 23(8):2154–2160. https://doi.org/10.1111/codi.15748
Vather R, Josephson R, Jaung R, Robertson J, Bissett I (2015) Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 157(4):764–773. https://doi.org/10.1016/j.surg.2014.12.005
Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S (2015) Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin 31(4):677–686. https://doi.org/10.1185/03007995.2015.1005833
Alhashemi M, Fiore JF Jr, Safa N et al (2019) Incidence and predictors of prolonged postoperative ileus after colorectal surgery in the context of an enhanced recovery pathway. Surg Endosc 33(7):2313–2322. https://doi.org/10.1007/s00464-018-6514-4
Clavien PA, Barkun J, De Oliveira ML et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2
Huisman DE, Reudink M, Van Rooijen SJ et al (2022) LekCheck: a prospective study to identify perioperative modifiable risk factors for anastomotic leakage in colorectal surgery. Ann Surg 275(1):e189–e197. https://doi.org/10.1097/SLA.0000000000003853
Nair VP, Hunter J (2004) Anticholinesterases and anticholinergic drugs. Contin Educ Anaesth Crit Care Pain 4(5):164–168. https://doi.org/10.1093/bjaceaccp/mkh045
Child CS (1984) Prevention of neostigmine-induced colonic activity. A comparison of atropine and glycopyrronium. Anaesthesia 39(11):1083–1085. https://doi.org/10.1111/j.1365-2044.1984.tb08927.x
Gallanosa A, Stevens J, Quick J (2021) Glycopyrrolate. In: StatPearls Publishing. Treasure Island (FL) https://www.ncbi.nlm.nih.gov/books/NBK526035/. Accessed 2021 Oct 11
Scarth E, Smith S (2016) Drugs in anaesthesia and intensive care. Oxford University Press, Oxford, UK
Bailey CR (2017) Sugammadex: when should we be giving it? Anaesthesia 72(10):1170–1175. https://doi.org/10.1111/anae.13960
Vaughan-Shaw PG, Fecher IC, Harris S, Knight JS (2012) A meta-analysis of the effectiveness of the opioid receptor antagonist alvimopan in reducing hospital length of stay and time to GI recovery in patients enrolled in a standardized accelerated recovery program after abdominal surgery. Dis Colon Rectum 55(5):611–620. https://doi.org/10.1097/DCR.0b013e318249fc78
Sapci I, Hameed I, Ceylan A et al (2020) Predictors of ileus following colorectal resections. Am J Surg 219(3):527–529. https://doi.org/10.1016/j.amjsurg.2019.10.002
Rao Kadam V, Howell S (2018) Unrestricted and restricted access to sugammadex and side effect profile in a teaching hospital Centre for year 2014- database audit study. Anesth Pain Med 8(1):e63066. https://doi.org/10.5812/aapm.63066
Traeger L, Koullouros M, Bedrikovetski S et al (2022) Cost of postoperative ileus following colorectal surgery: a cost analysis in the Australian public hospital setting. Colorectal Dis Early View. https://doi.org/10.1111/codi.16235
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. LT received grants from Royal Adelaide Hospital Colorectal Research Group and Dawes Scholarships, and University of Adelaide Research Training Program Stipend (a1175080).
Author information
Authors and Affiliations
Contributions
LT: conceptualisation; methodology; investigation; formal analysis; writing original draft. TDH: investigation; writing original draft; writing review and editing. SB: formal analysis; writing review and editing. HK: investigation; writing review and editing. NNDV: investigation; writing review and editing. JM: supervision; writing review and editing. TS: supervision; writing review and editing. Podium presentation at General Surgeons Australia Virtual Paper Day, Adelaide, December 2021, Royal Australasian College of Surgeons (RACS) Annual Scientific Congress, Brisbane, May 2022, and RACS RP Jepson Paper Day, Adelaide, August 2022.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Central Adelaide Local Health Network Human Research Ethics Committee.
Informed consent
A waiver of consent for retrospective patients was provided in accordance with the guidelines provided by the National Health and Medical Research Council’s (NHMRC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Traeger, L., Hall, T.D., Bedrikovetski, S. et al. Effect of neuromuscular reversal with neostigmine/glycopyrrolate versus sugammadex on postoperative ileus following colorectal surgery. Tech Coloproctol 27, 217–226 (2023). https://doi.org/10.1007/s10151-022-02695-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-022-02695-w