Abstract
Background
Platinum/taxane (TC) chemotherapy with debulking surgery stays the mainstay of the treatment in ovarian cancer patients with peritoneal metastasis, and recently its novel modality, intraperitoneal carboplatin with dose-dense paclitaxel (ddTCip), was shown to have greater therapeutic impact. Nevertheless, the response varies among patients and consequent recurrence, or relapse often occurs. Discovery of therapeutic response predictor to ddTCip and/or TC therapy is eagerly awaited to improve the treatment outcome.
Methods
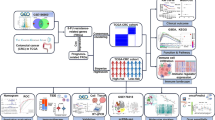
Using datasets in 76 participants in our ddTCip study and published databases on patients received TC therapy, we first validated a total of 75 previously suggested markers, sought out more active biomarkers through the association analyses of genome-wide transcriptome and genotyping data with progression-free survival (PFS) and adverse events, and then developed multiplex statistical prediction models for PFS and toxicity by mainly using multiple regression analysis and the classification and regression tree (CART) algorithm.
Results
The association analyses revealed that SPINK1 could be a possible biomarker of ddTCip efficacy, while ABCB1 rs1045642 and ERCC1 rs11615 would be a predictor of hematologic toxicity and peripheral neuropathy, respectively. Multiple regression analyses and CART algorithm finally provided a potent efficacy prediction model using 5 gene expression data and robust multiplex toxicity prediction models-CART models using a total of 4 genotype combinations and multiple regression models using 15 polymorphisms on 12 genes.
Conclusion
Biomarkers and multiplex models composed here could work well in the response prediction of ddTCip and/or TC therapy, which might contribute to realize optimal selection of the key therapy.




Similar content being viewed by others
References
Torre LA, Trabert B, DeSantis CE et al (2018) Ovarian cancer statistics. CA Cancer J Clin 68(4):284–296. https://doi.org/10.3322/caac.21456
Jayson GC, Kohn EC, Kitchener HC et al (2014) Ovarian cancer. Lancet 384(9951):1376–1388. https://doi.org/10.1016/S0140-6736(13)62146-7
Sambasivan S (2022) Epithelial ovarian cancer: Review article. Cancer Treat Res Commun. 33:100629. https://doi.org/10.1016/j.ctarc.2022.100629
Hasegawa K, Shimada M, Takeuchi S et al (2020) A phase II study of intraperitoneal carboplatin plus intravenous dose-dense paclitaxel in front-line treatment of suboptimal residual ovarian cancer. Br J Cancer 122(6):766–770. https://doi.org/10.1038/s41416-020-0734-9
Nagao S, Fujiwara K, Yamamoto K et al (2023) Intraperitoneal carboplatin for ovarian cancer- a phase 2/3 trial. NEJM Evid. https://doi.org/10.1056/EVIDoa2200225
Aronson SL, Lopez-Yurda M, Koole SN, et al. (2023) Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian cancer (OVHIPEC-1): final survival analysis of a randomised, controlled, phase 3 trial Lancet Oncol. 24(10):1109–1118, https://doi.org/10.1016/S1470-2045(23)00396-0.
Medeiros R, Pereira D, Afonso N et al (2003) Platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma: glutathione S-transferase genetic polymorphisms as predictive biomarkers of disease outcome. Int J Clin Oncol 8(3):156–161. https://doi.org/10.1007/s10147-003-0318-8
Komatsu M, Hiyama K, Tanimoto K et al (2006) Prediction of individual response to platinum/paclitaxel combination using novel marker genes in ovarian cancers. Mol Cancer Ther 5(3):767–775. https://doi.org/10.1158/1535-7163.MCT-05-0408
Ferrandina G, Zannoni GF, Martinelli E et al (2006) Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res 12(9):2774–2779. https://doi.org/10.1158/1078-0432.CCR-05-2715
Spratlin J, Sawyer MB (2007) Pharmacogenetics of paclitaxel metabolism. Crit Rev Oncol Hematol 61(3):222–229. https://doi.org/10.1016/j.critrevonc.2006.09.006
Bonome T, Levine DA, Shih J et al (2008) A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res 68(13):5478–5486. https://doi.org/10.1158/0008-5472.CAN-07-6595
Marsh S, McLeod H, Dolan E et al (2009) Platinum pathway. Pharmacogenet Genomics 19(7):563–564. https://doi.org/10.1097/FPC.0b013e32832e0ed7
Cancer Genome Atlas Research Network (2011) Bell D, Berchuck A, Birrer M, et al. Integrated genomic analyses of ovarian carcinoma Nature. 474(7353):609–615. https://doi.org/10.1038/nature10166
Gillet JP, Calcagno AM, Varma S et al (2012) Multidrug resistance-linked gene signature predicts overall survival of patients with primary ovarian serous carcinoma. Clin Cancer Res 18(11):3197–3206. https://doi.org/10.1158/1078-0432.CCR-12-0056
Bosquet JG, Marchion DC, Chon H et al (2014) Analysis of chemotherapeutic response in ovarian cancers using publicly available high-throughput data. Cancer Res 74(14):3902–3912. https://doi.org/10.1158/0008-5472.CAN-14-0186
Murakami R, Matsumura N, Brown JB et al (2016) Prediction of taxane and platinum sensitivity in ovarian cancer based on gene expression profiles. Gynecol Oncol 141(1):49–56. https://doi.org/10.1016/j.ygyno.2016.02.027
Sanchez-Vega F, Mina M, Armenia J et al (2018) Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173(2):321–37.e10. https://doi.org/10.1016/j.cell.2018.03.035
Heitz F, Kommoss S, Tourani R et al (2020) Dilution of molecular-pathologic gene signatures by medically associated factors might prevent prediction of resection status after debulking surgery in patients with advanced ovarian cancer. Clin Cancer Res 26(1):213–219. https://doi.org/10.1158/1078-0432.CCR-19-1741
Zehra A, Zehra S, Ismail M et al (2018) Glutathione S-transferase M1 and T1 gene deletions and susceptibility to acute lymphoblastic leukemia (ALL) in adults. Pak J Med Sci 34(3):666–670. https://doi.org/10.12669/pjms.343.14911
Wu G, Peng H, Tang M et al (2021) ZNF711 down-regulation promotes CISPLATIN resistance in epithelial ovarian cancer via interacting with JHDM2A and suppressing SLC31A1 expression. EBioMedicine 71:103558. https://doi.org/10.1016/j.ebiom.2021.103558
Yu H, Wang H, Qie A et al (2021) FGF13 enhances resistance to platinum drugs by regulating hCTR1 and ATP7A via a microtubule-stabilizing effect. Cancer Sci 112(11):4655–4668. https://doi.org/10.1111/cas.15137
Zhang Y, Cao S, Zhuang C et al (2021) ERCC1 rs11615 polymorphism and chemosensitivity to platinum drugs in patients with ovarian cancer: a systematic review and meta-analysis. J Ovarian Res 14(1):80. https://doi.org/10.1186/s13048-021-00831-y
Yamamoto Y. Komatsu M, Fumoto S, et al. Selection of novel maker genes that predict a response to paclitaxel and carboplatin (TC) therapy in ovarian cancer. Cancer Res. 2009 May; 69(9_Supplement), 892. (2009 AACR Annual Meeting Abstract #892)
Mehner C, Oberg AL, Kalli KR et al (2015) Serine protease inhibitor Kazal type 1 (SPINK1) drives proliferation and anoikis resistance in a subset of ovarian cancers. Oncotarget 6(34):35737–35754. https://doi.org/10.18632/oncotarget.5927
Mehner C, Miller E, Hockla A et al (2020) Targeting an autocrine IL-6-SPINK1 signaling axis to suppress metastatic spread in ovarian clear cell carcinoma. Oncogene 39(42):6606–6618. https://doi.org/10.1038/s41388-020-01451-4
Lin T-C (2021) Functional roles of SPINK1 in cancers. Int J Mol Sci 22(8):3814. https://doi.org/10.3390/ijms22083814
Chen F, Long Q, Fu D et al (2018) Targeting SPINK1 in the damaged tumour microenvironment alleviates therapeutic resistance. Nat Commun 9(1):4315. https://doi.org/10.1038/s41467-018-06860-4
Liao C, Wang Q, An J et al (2022) SPINKs in tumors: potential therapeutic targets. Front Oncol 12:833741. https://doi.org/10.3389/fonc.2022.833741
Cecchin E, Stocco G (2020) Pharmacogenomics and personalized medicine. Genes (Basel) 11(6):679. https://doi.org/10.3390/genes11060679
Chang WC, Tanoshima R, Ross CJD et al (2021) Challenges and opportunities in implementing pharmacogenetic testing in clinical settings. Annu Rev Pharmacol Toxicol 61:65–84. https://doi.org/10.1146/annurev-pharmtox-030920-025745
Sissung TM, Mross K, Steinberg SM et al (2006) Association of ABCB1 genotypes with paclitaxel-mediated peripheral neuropathy and neutropenia. Eur J Cancer 42(17):2893–2896. https://doi.org/10.1016/j.ejca.2006.06.017
Inada M, Sato M, Morita S et al (2010) Associations between oxaliplatin-induced peripheral neuropathy and polymorphisms of the ERCC1 and GSTP1 genes. Int J Clin Pharmacol Ther 48(11):729–734. https://doi.org/10.5414/cpp48729
Zhang H, Liu T, Zhang Z et al (2016) Integrated Proteogenomic characterization of human high-grade serous ovarian cancer. Cell 166(3):755–765. https://doi.org/10.1016/j.cell.2016.05.069
Bhardwaj A, Josse C, Van Daele D et al (2022) Deeper insights into long-term survival heterogeneity of pancreatic ductal adenocarcinoma (PDAC) patients using integrative individual- and group-level transcriptome network analyses. Sci Rep 12(1):11027. https://doi.org/10.1038/s41598-022-14592-1
Gong J, Zhou Y, Liu D et al (2018) F-box proteins involved in cancer-associated drug resistance. Oncol Lett 15(6):8891–8900. https://doi.org/10.3892/ol.2018.8500
Xiang Y, Wang W, Gu J et al (2022) Circular RNA VANGL1 facilitates migration and invasion of papillary thyroid cancer by modulating the miR-194/ZEB1/EMT axis. J Oncol. https://doi.org/10.1155/2022/4818651
Xie J, Ye J, Cai Z et al (2020) GPD1 Enhances the anticancer effects of metformin by synergistically increasing total cellular Glycerol-3-phosphate. Cancer Res 80(11):2150–2162. https://doi.org/10.1158/0008-5472.CAN-19-2852
Wenlong Zhang W, He X, Yin H et al (2022) Allosteric activation of the metabolic enzyme GPD1 inhibits bladder cancer growth via the lysoPC-PAFR-TRPV2 axis. J Hematol Oncol 15(1):93. https://doi.org/10.1186/s13045-022-01312-5
Garvie CW, Wu X, Papanastasiou M et al (2021) Structure of PDE3A-SLFN12 complex reveals requirements for activation of SLFN12 RNase. Nat Commun 12(1):4375. https://doi.org/10.1038/s41467-021-24495-w
Hao N, Shen W, Du R et al (2020) Phosphodiesterase 3A represents a therapeutic target that drives stem cell-like property and metastasis in breast cancer. Mol Cancer Ther 19(3):868–881. https://doi.org/10.1158/1535-7163.MCT-18-1233
de Waal L, Lewis TA, Rees MG et al (2016) Identification of cancer-cytotoxic modulators of PDE3A by predictive chemogenomics. Nat Chem Biol 12(2):102–108. https://doi.org/10.1038/nchembio.1984
Koepsell H (2017) The Na+-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Ther 170:148–165. https://doi.org/10.1016/j.pharmthera.2016.10.017
Lai B, Xiao Y, Pu H et al (2012) Overexpression of SGLT1 is correlated with tumor development and poor prognosis of ovarian carcinoma. Arch Gynecol Obstet 285(5):1455–1461. https://doi.org/10.1007/s00404-011-2166-5
Walker JL, Brady MF, Wenzel L et al (2019) Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG oncology/gynecologic oncology group study. J Clin Oncol 37(16):1380–1390. https://doi.org/10.1200/JCO.18.01568
Gandara DR, Kawaguchi T, Crowley J et al (2009) Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol 27(21):3540–3546. https://doi.org/10.1200/JCO.2008.20.8793
Komatsu M, Wheeler HE, Chung S et al (2015) Pharmacoethnicity in paclitaxel-induced sensory peripheral neuropathy. Clin Cancer Res 21(19):4337–4346. https://doi.org/10.1158/1078-0432.CCR-15-0133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kawabata-Iwakawa, R., Iwasa, N., Satoh, K. et al. Prediction of response to promising first-line chemotherapy in ovarian cancer patients with residual peritoneal tumors: practical biomarkers and robust multiplex models. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02552-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02552-w