Abstract
Background
Skeletal muscle (SM) is a key factor in cancer treatment. However, it is unclear whether pretreatment SM change affects the outcome of immune checkpoint inhibitors (ICIs) therapy in gastric cancer (GC).
Methods
Advanced GCs treated with ICIs were retrospectively investigated. SM evaluated by psoas muscle area at the third lumbar vertebra was measured on CT acquired within 1 month from the start of ICIs therapy (CT-1), and on CT acquired 2.8 ± 0.84 months before CT-1. Monthly change rate of SM (MCR-SM) was defined as the change rate of SMs between those two CTs divided by the period between those CTs (month). Monthly change rate of body weight (MCR-BW) during the same period was also calculated. They were compared with disease-specific survival (DSS) and progression-free survival (PFS). MCR-SM was compared with pretreatment markers including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), C-reactive protein (CRP), and liver-to-spleen CT attenuation ratio (LSR) as a marker of liver lipid metabolism.
Results
This study enrolled eighty-three GC patients. MCR-SM significantly correlated with DSS and PFS (P < 0.0001, 0.001, respectively), whereas MCR-BW did not. Kaplan–Meier analyses demonstrated that higher MCR-SM (MCR-SM ≥ −0.7185%) significantly associated with better DSS and PFS (P = 0.0002, 0.03, respectively). Patients with positive MCR-SM showed significantly lower NLR, MLR, and CRP than those with negative (P = 0.01, 0.006, 0.003, respectively). MCR-SM showed a significant positive correlation with LSR (P = 0.007, R = 0.30).
Conclusions
Pretreatment SM loss, associated with high systemic inflammation and hepatic fat accumulation, related to poor outcome of ICIs therapy in GC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth common cancer and the third leading cause of cancer-related death worldwide [1]. Since the ATTRACTION-2 study [2] and the KEYNOTE-059 study [3], immune checkpoint inhibitors (ICIs) have been recommended for previously treated unresectable advanced or recurrent gastric cancer. However, those studies reported that the 1-year overall survival (OS) rate of ICIs therapy, such as nivolumab and pembrolizumab, was 23.2–26.2% [2, 3]. It means that about 75% of patients die within 1 year, although ICIs therapy is obviously better than placebo. Therefore, investigation of biomarkers or clues to improve treatment response of ICIs are highly desirable.
On the other hand, it has been reported that cachexia is associated with poor prognosis in any types of cancer, and a typical symptom of cachexia is loss of skeletal muscle (SM) [4]. In nivolumab treatment of advanced GC patients, a recent study reported that the measurement of SM mass index at the time of starting ICIs therapy might be useful for predicting treatment outcome [5]. However, it is still unclear whether the degree of SM change before ICIs therapy can predict treatment outcome of ICIs therapy for advanced GC. If SM change before ICIs therapy really has an impact on the outcome of ICIs therapy, we may be able to find a new strategy to enhance ICIs therapy from the viewpoint of SM intervention.
Therefore, this study investigated whether SM change before ICIs therapy was associated with prognosis of ICIs therapy in advanced GC, compared with various cachexia related markers, such as systemic inflammatory markers or liver lipid metabolism [6,7,8].
Materials and methods
Patient population
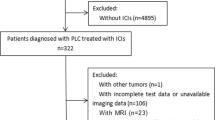
This retrospective study was performed under the approval of the institutional review board at our institute (IRB No. 3032). Written informed consent for participation was waived because of the retrospective nature of this study. We retrospectively identified unresectable advanced or recurrent gastric cancer patients treated with ICIs (nivolumab or pembrolizumab) in our hospital from October 2017 to June 2023. All patients received at least first-line chemotherapy prior to ICIs therapy.
Treatment and follow-up
The treatment schedule and the dose modification schema of ICIs therapy using nivolumab and pembrolizumab have been detailed previously [2, 9]. Nivolumab was administered intravenously at a dose of 240 mg every 2 weeks, and pembrolizumab was administered intravenously at a dose of 200 mg every 3 weeks. These treatments were continued until disease progression, unacceptable toxicity, or patient refusal. Tumor responses were assessed by CT every 2–4 cycles of ICIs therapy. After the failure of ICIs therapy, any additional treatment occurred at the discretion of the treating physician, but basically conformed to the Japanese gastric cancer treatment guidelines [10].
Assessment of skeletal muscle change and body weight change
Patients were examined on 320/80-section multi-detector row CT (MDCT) scanners (Aquilion ONE VISION/Aquilion PRIME; Canon Medical Systems, Otawara, Japan). The following CT parameters were used for acquisition of volume data: 100 kVp; 150–200 mA; 0.5-s rotation time; field of view, 360; matrix, 512; pixel size, 0.7 mm × 0.7 mm; 5 mm reconstructed slice thickness. SM was evaluated by the areas of psoas muscle (PM) at the third lumbar vertebra on CT image, and this PM area within a range of −29 to 150 Hounsfield units was automatically identified using Synapse Vincent (Fujifilm Inc., Tokyo, Japan) (Fig. 1). PM was measured on CT acquired within 1 month from the start of ICIs therapy (CT-1), and on CT acquired at 2.8 ± 0.84 months before CT-1. Monthly change rate of SM (MCR-SM) was defined as the change rate (%) of PMs between those two CTs at different timepoints divided by the period between those CTs (month). Monthly change rate of body weight (MCR-BW) was also calculated as the change rate of body weights between those two CTs divided by the period between those CTs (month).
Systemic inflammatory markers’ measurements before ICIs therapy
Systemic inflammatory markers were calculated as following; neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing absolute neutrophil count by lymphocyte count, platelet-to-lymphocyte ratio (PLR) was by dividing thrombocyte count by lymphocyte count, and monocyte-to-lymphocyte ratio (MLR) was by dividing monocyte count by lymphocyte count measured in peripheral blood before ICIs therapy. Serum C-reactive protein (CRP) was also measured in peripheral blood before ICIs therapy.
Assessment of liver lipid metabolism
Liver lipid metabolism was assessed by non-contrast enhanced CT acquired within 1 month from the start of ICIs therapy (CT-1). Mean CT attenuation values were obtained for both the liver and the spleen using a single oval region-of-interest (ROI) of about 200 mm2, taking care to avoid vessels, calcifications, and masses, if present. Thereafter, liver-to-spleen ratio (LSR; liver attenuation/spleen attenuation) was calculated as the quantitative marker for liver lipid metabolism [11, 12].
Statistical analysis
Statistical analyses were performed using the JMP Pro 16.0 (SAS Institute, Inc., Cary, NC, USA), and for all comparisons, P < 0.05 was considered to indicate a statistically significant difference. The associations of the continuous variables with disease-specific survival (DSS) and progression-free survival (PFS) after the ICIs therapy were evaluated using the Cox proportional hazards regression model. Kaplan–Meier analysis was also performed for DSS and PFS analyses, and the log-rank test was employed. The Mann–Whitney U test was applied for comparison of inflammatory markers between positive MCR-SM and negative MCR-SM groups. Correlations between MCR-SM and LSR was analyzed using Spearman’s rank correlation coefficients.
Results
Patient characteristics
Eighty-three patients who were treated with ICIs in our hospital were eligible for this study. This subject included 56 men and 27 women, with a median age of 70.0 years (range 23–87 years). Seventy-nine cases were treated with Nivolumab, while 4 cases were treated with Pembrolizumab. Patients’ characteristics and their associations with MCR-SM are summarized in Table 1. Higher MCR-SM was significantly associated with older age (≥71), recurrence GC rather than unresectable GC, higher BMI at ICIs therapy (≥19.72%), higher MCR-BW (≥ −0.231%), IrAEs occurrence, higher serum albumin (≥3.4), and higher prognostic nutrition index (≥ 39.815) (P = 0.006, 0.03, 0.008, 0.01, 0.01, 0.004, 0.008, respectively).
Correlations of skeletal muscle change with survival
In univariate Cox regression analysis (Table 2), MCR-SM showed significant correlations with DSS and PFS (P < 0.0001, 0.001, respectively), but MCR-BW did not (P = 0.08, 0.2, respectively). Kaplan–Meier analyses demonstrated that patients with higher MCR-SM (MCR-SM ≥ −0.7185%, median value) had significantly better DSS and PFS (P = 0.0002, 0.03, respectively; Fig. 2).
Associations of skeletal muscle change with systemic inflammatory markers and liver lipid metabolism
This study cohort included 33 patients with positive MCR-SM (MCR-SM ≥ 0%) and 50 with negative MCR-SM (MCR-SM < 0%). Patients with positive MCR-SM showed significantly lower NLR, MLR, and CRP values than those with negative MCR-SM (P = 0.01, 0.006, 0.003, respectively), whereas PLR did not show a significant association with MCR-SM (P = 0.4, Fig. 3). MCR-SM showed a significant positive correlation with LSR (P = 0.007, R = 0.30; Fig. 4.).
Discussion
A meta-analysis including 2501 patients with solid cancers reported that sarcopenia could predict the response to ICIs and survival after ICIs therapy [13]. Another meta-analysis demonstrated that not only the baseline sarcopenia but the development or worsening of sarcopenia during immunotherapy associated with poor overall survival in advanced non-small cell lung cancer [14]. In ICIs therapy of GC, three previous studies reported that low baseline SM or sarcopenia was associated with poor survival after ICIs therapy, and they are summarized in Table 3 [5, 15, 16]. Therefore, SM loss, which is a typical symptom of cachexia, obviously plays an important role in ICIs therapy. However, these previous studies focused on SM index or PM index at the time of starting ICIs therapy, or the change of SM or PM index “after” ICIs therapy, and it was still unclear whether the degree of SM change prior to ICIs therapy affected the outcome of ICIs therapy. In this context, our study demonstrated that SM change rate before ICIs therapy obviously affected the outcome of ICIs therapy. Considering these results including ours, prevention of SM loss before and during ICIs therapy is quite important to make ICIs therapy more effective. The prognostic importance of SM loss was also reported in the first-line chemotherapy of GC [15]; therefore, we strongly recommend keeping SM in both immunotherapy and cytotoxic chemotherapy of GC with an intervention on SM or nutrition approach. Furthermore, a meta-analysis demonstrated that preoperative SM loss increased the risk of postoperative complications and reduced the overall survival rate of patients undergoing gastrectomy for GC [17]. Of course, some patients with severe cachexia such as refractory cachexia, which was categorized by Fearon et al. in 2011 [18], might be resistance to exercise or nutrition intervention, because it was reported that those at refractory cachexia were resistant to any type of intervention due to strong catabolic effects [19]. However, it is still difficult to precisely distinguish patients with refractory cachexia from those with other cachexia categories such as precachexia and cachexia who may have a chance to improve their prognosis with exercise or nutrition intervention to keep SM [20,21,22]; therefore, it would be better for all GC patients to start exercise or nutrition intervention to prevent SM loss as soon as they are diagnosed as GC at any stage, if they want to improve their treatment outcome.
This study also compared SM change with systemic inflammatory markers and liver lipid metabolism, and demonstrated that short-term SM loss prior to ICIs therapy was associated with high systemic inflammation and hepatic fat accumulation (lower LSR) before ICIs therapy. Generally, inflammatory markers, such as NLR, PLR, LMR, and CRP, were reported their associations with cancer cachexia [23,24,25], and some previous reports demonstrated that the elevations of these inflammatory markers were associated with poor outcome of cancer patients treated with ICIs therapy [26,27,28,29]. On the other hand, our previous study demonstrated that ICIs were ineffective in GC patients with severe hepatic steatosis [6]. Similarly, Pfister et al. [30] reported that ICIs were ineffective in non-alcoholic steatohepatitis (NASH)-related hepatocellular carcinoma. Pfister et al. [30] suggested that NASH-related aberrant T cell activation caused tissue damage leading to impaired immune surveillance. Our previous study suggested that hepatic steatosis was associated with cancer cachexia leading to poor outcome in ICIs therapy [6]. Considering the results of these previous reports, it was reasonable that SM loss before ICIs therapy, which had significant associations with high systemic inflammation and hepatic fat accumulation, showed a significant correlation with worse survival in ICIs therapy of GC. However, further investigation will be needed to confirm this relationship.
Our study has several limitations. First, our findings are based on single-center data, and the sample size was small; therefore, this study should be validated in a larger patient population. Second, the period to measure SM change was varied in each individual case, due to the retrospective nature of this study, which can be a bias of this study. Third, ROIs for liver and spleen were manually drawn, and this procedure for measurement of LSR might be relatively subjective. A computerized new segmentation method with high reproducibility and reliability should be investigated in further studies.
Conclusion
Though this was a relatively small study, it was demonstrated that short-term SM loss prior to ICIs therapy was associated with poor survival in GC patients. Exercise or nutrition intervention to prevent SM loss before ICIs therapy might improve the outcome of ICIs therapy. We believe that our results will provide an important insight into selecting the optimal therapeutic strategy for patients with advanced GCs.
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Kang Y-K, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461–2471. https://doi.org/10.1016/S0140-6736(17)31827-5
Fuchs CS, Doi T, Jang RW et al (2018) Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 4:e180013. https://doi.org/10.1001/jamaoncol.2018.0013
Fearon K, Arends J, Baracos V (2013) Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 10:90–99. https://doi.org/10.1038/nrclinonc.2012.209
Kano M, Hihara J, Tokumoto N et al (2021) Association between skeletal muscle loss and the response to nivolumab immunotherapy in advanced gastric cancer patients. Int J Clin Oncol 26:523–531. https://doi.org/10.1007/s10147-020-01833-4
Hayano K, Ohira G, Kano M et al (2023) Prognostic impact of hepatic steatosis evaluated by CT on immunotherapy for gastric cancer: associations with sarcopenia, systemic inflammation, and hormones. Oncology 101:185–192. https://doi.org/10.1159/000528005
Rohm M, Zeigerer A, Machado J et al (2019) Energy metabolism in cachexia. EMBO Rep 20:e47258. https://doi.org/10.15252/embr.201847258
Webster JM, Kempen LJAP, Hardy RS et al (2020) Inflammation and skeletal muscle wasting during cachexia. Front Physiol 11:597675. https://doi.org/10.3389/fphys.2020.597675
Shitara K, Özgüroğlu M, Bang Y-J et al (2018) Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392:123–133. https://doi.org/10.1016/S0140-6736(18)31257-1
Japanese Gastric Cancer Association (2023) Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 26:1–25. https://doi.org/10.1007/s10120-022-01331-8
Boyce CJ, Pickhardt PJ, Kim DH et al (2010) Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 194:623–628. https://doi.org/10.2214/AJR.09.2590
Graffy PM, Pickhardt PJ (2016) Quantification of hepatic and visceral fat by CT and MR imaging: relevance to the obesity epidemic, metabolic syndrome and NAFLD. Br J Radiol 89:20151024. https://doi.org/10.1259/bjr.20151024
Takenaka Y, Oya R, Takemoto N et al (2021) Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle 12:1122–1135. https://doi.org/10.1002/jcsm.12755
Wang J, Cao L, Xu S (2020) Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol 88:106907. https://doi.org/10.1016/j.intimp.2020.106907
Park SE, Choi JH, Park JY et al (2020) Loss of skeletal muscle mass during palliative chemotherapy is a poor prognostic factor in patients with advanced gastric cancer. Sci Rep 10:17683. https://doi.org/10.1038/s41598-020-74765-8
Fujii H, Makiyama A, Iihara H et al (2020) Cancer cachexia reduces the efficacy of nivolumab treatment in patients with advanced gastric cancer. Anticancer Res 40:7067–7075. https://doi.org/10.21873/anticanres.14734
Chen F, Chi J, Liu Y et al (2022) Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: a meta-analysis of cohort studies. Arch Gerontol Geriatr 98:104534. https://doi.org/10.1016/j.archger.2021.104534
Fearon K, Strasser F, Anker SD et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Watanabe H, Oshima T (2023) The latest treatments for cancer cachexia: an overview. Anticancer Res 43:511–521. https://doi.org/10.21873/anticanres.16188
Garcia DO, Thomson CA (2014) Physical activity and cancer survivorship. Nutr Clin Pract 29:768–779. https://doi.org/10.1177/0884533614551969
Rock CL, Thomson CA, Sullivan KR et al (2022) American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin 72:230–262. https://doi.org/10.3322/caac.21719
Yang L, Morielli AR, Heer E et al (2021) Effects of exercise on cancer treatment efficacy: a systematic review of preclinical and clinical studies. Cancer Res 81:4889–4895. https://doi.org/10.1158/0008-5472.CAN-21-1258
Kasprzak A (2021) The role of tumor microenvironment cells in colorectal cancer (CRC) cachexia. Int J Mol Sci 22:1565. https://doi.org/10.3390/ijms22041565
McGovern J, Dolan RD, Skipworth RJ et al (2022) Cancer cachexia: a nutritional or a systemic inflammatory syndrome? Br J Cancer 127:379–382. https://doi.org/10.1038/s41416-022-01826-2
Roxburgh CSD, McMillan DC (2014) Cancer and systemic inflammation: treat the tumour and treat the host. Br J Cancer 110:1409–1412. https://doi.org/10.1038/bjc.2014.90
Cupp MA, Cariolou M, Tzoulaki I et al (2020) Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 18:360. https://doi.org/10.1186/s12916-020-01817-1
Ogata T, Satake H, Ogata M et al (2018) Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget 9:34520–34527. https://doi.org/10.18632/oncotarget.26145
Sato S, Oshima Y, Matsumoto Y et al (2021) The new prognostic score for unresectable or recurrent gastric cancer treated with nivolumab: a multi-institutional cohort study. Ann Gastroenterol Surg 5:794–803. https://doi.org/10.1002/ags3.12489
Xu H, He A, Liu A et al (2019) Evaluation of the prognostic role of platelet-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int Immunopharmacol 77:105957. https://doi.org/10.1016/j.intimp.2019.105957
Pfister D, Núñez NG, Pinyol R et al (2021) NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 592:450–456. https://doi.org/10.1038/s41586-021-03362-0
Acknowledgements
This study has no specific grant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or other relations that could lead to a conflict of interest.
Compliance with ethical standards
The authors declare that they have no conflicts of interest. This retrospective study protocol was approved by the institutional review board at our institute (IRB No. 3032). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent for participation was not required because of the retrospective nature of this study.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hayano, K., Ohira, G., Matsumoto, Y. et al. CT-derived skeletal muscle change before immunotherapy predicts survival of advanced gastric cancer: associations with inflammatory markers and liver lipid metabolism. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02551-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02551-x