Abstract
Background
As a substantial waiting time is usually required for radical surgery, safe and effective preoperative neoadjuvant chemotherapy (NAC) is desired for the treatment of locally advanced head and neck squamous cell carcinoma (HNSCC). However, the significance of NAC in advanced HNSCC is still unclear. This study aimed to assess the safety and efficacy of NAC using the paclitaxel, carboplatin, and cetuximab (PCE) regimen.
Methods
We retrospectively evaluated the background characteristics, incidence of adverse events, overall response rate (ORR), pathological response, recurrence-free survival (RFS), and overall survival (OS) in 26 patients. Patients receiving the PCE regimen were further divided into two groups based on the number of chemotherapy cycles (one cycle or more) and eligibility for cisplatin. Patients aged ≥ 75 years and those with an estimated glomerular filtration rate (eGFR) < 60 mL/min were classified as ineligible for cisplatin.
Results
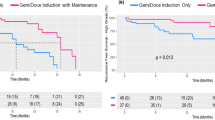
The median age was 70 (27–81) years. The median eGFR at treatment initiation was 63.2 (41.1–89.7) mL/min. Fourteen (53.8%) patients were ineligible for cisplatin. Grade 3 or higher neutropenia was observed in 11 of 25 (42.3%) patients. No delay in or withdrawal from surgery was observed. The ORR was 65.4%. The 2-year RFS and OS were 61.5% and 76.7%, respectively. No significant differences in safety and efficacy between the number of chemotherapy cycles and cisplatin eligibility were observed.
Conclusion
NAC using the PCE regimen for patients with locally advanced HNSCC, including cisplatin-ineligible patients, has acceptable toxicity and favorable efficacy.




Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
References
National Comprehensive Cancer Network. Head and neck cancers, version 2.2023. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf Accessed 23 Aug 2023
Xiao R, Ward MC, Yang K et al (2018) Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: a mechanism for increased mortality. Cancer 124:1400–1414. https://doi.org/10.1002/cncr.31213. (PMID: 29315499)
Liao DZ, Schlecht NF, Rosenblatt G et al (2019) Association of delayed time to treatment initiation with overall survival and recurrence among patients with head and neck squamous cell carcinoma in an underserved urban population. JAMA Otolaryngol Head Neck Surg 145:1001–1009. https://doi.org/10.1001/jamaoto.2019.2414. (PMID: 31513264)
Schoonbeek RC, Zwertbroek J, Plaat BEC et al (2021) Determinants of delay and association with outcome in head and neck cancer: a systematic review. Eur J Surg Oncol 47:1816–1827. https://doi.org/10.1016/j.ejso.2021.02.029. (PMID: 33715909)
Rygalski CJ, Zhao S, Eskander A et al (2021) Time to surgery and survival in head and neck cancer. Ann Surg Oncol 28:877–885. https://doi.org/10.1245/s10434-020-09326-4
Brody RM, Albergotti WG, Shimunov D et al (2020) Changes in head and neck oncologic practice during the COVID-19 pandemic. Head Neck 42:1448–1453. https://doi.org/10.1002/hed.26233
Ando N, Kato H, Igaki H et al (2011) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74. https://doi.org/10.1245/s10434-011-2049-9
Allum WH, Stenning SP, Bancewicz J et al (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27:5062–5067. https://doi.org/10.1200/JCO.2009.22.2083
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785. https://doi.org/10.1200/JCO.2007.15.0235
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19:27–39. https://doi.org/10.1016/S1470-2045(17)30777-5
Kitamura H, Tsukamoto T, Shibata T et al (2014) Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan clinical oncology group study JCOG0209. Ann Oncol. https://doi.org/10.1093/annonc/mdu126
International Collaboration of Trialists (2011) International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 29:2171–2177. https://doi.org/10.1200/JCO.2010.32.3139
Vermorken JB, Remenar E, van Herpen C et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704. https://doi.org/10.1056/NEJMoa071028
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715. https://doi.org/10.1056/NEJMoa070956
Licitra L, Grandi C, Guzzo M et al (2013) Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial. J Clin Oncol 21:327–333. https://doi.org/10.1200/JCO.2003.06.146
Zhong LP, Zhang CP, Ren GX et al (2013) Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol 31:744–751. https://doi.org/10.1200/JCO.2012.43.8820
Takenaka M, Arai A, Yoshizaka K et al (2019) Feasibility of combination of paclitaxel, carboplatin, and cetuximab as induction chemotherapy for advanced head and neck squamous cell carcinoma. Clin Oncol 4:1657. https://doi.org/10.25107/2474-1663.1657
Enokida T, Ogawa T, Homma A et al (2020) A multicenter phase II trial of paclitaxel, carboplatin, and cetuximab followed by chemoradiotherapy in patients with unresectable locally advanced squamous cell carcinoma of the head and neck. Cancer Med 9:1671–1682. https://doi.org/10.1002/cam4.2852
Kies MS, Holsinger FC, Lee JJ et al (2010) Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 28:8–14. https://doi.org/10.1200/JCO.2009.23.0425
Tahara M, Kiyota N, Yokota T et al (2018) Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol 29:1004–1009. https://doi.org/10.1093/annonc/mdy040
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Japan Society for Head and Neck Cancer (2018) General rules for clinical studies on head and neck cancer, 6th edn. Kanehara & Co., Ltd
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Sun L, Candelieri-Surette D, Anglin-Foote T et al (2022) Cetuximab-based vs carboplatin-based chemoradiotherapy for patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg 148:1022–1028. https://doi.org/10.1001/jamaoto.2022.2791
Haddad RI, Massarelli E, Lee JJ et al (2019) Weekly paclitaxel, carboplatin, cetuximab, and cetuximab, docetaxel, cisplatin, and fluorouracil, followed by local therapy in previously untreated, locally advanced head and neck squamous cell carcinoma. Ann Oncol 30:471–477. https://doi.org/10.1093/annonc/mdy549
Shirasu H, Yokota T, Kawakami T et al (2020) Efficacy and feasibility of induction chemotherapy with paclitaxel, carboplatin and cetuximab for locally advanced unresectable head and neck cancer patients ineligible for combination treatment with docetaxel, cisplatin, and 5-fluorouracil. Int J Clin Oncol 25:1914–1920. https://doi.org/10.1007/s10147-020-01742-6
Bang J, Shin HI, Kim GJ et al (2023) Oncologic and functional outcomes of neoadjuvant chemotherapy followed by surgery in human papillomavirus-positive tonsillar cancer. Head Neck 45:2580–2588. https://doi.org/10.1002/hed.27482
Chaukar D, Prabash K, Rane P et al (2022) Prospective phase II open-label randomized controlled trial to compare mandibular preservation in upfront surgery with neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. J Clin Oncol 40:272–281. https://doi.org/10.1200/JCO.21.00179
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing services.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
Conception and design: R. Tanaka, Y. Ueki, T. Takahashi, and A. Horii. Data analysis and interpretation: R. Tanaka, Y. Ueki, S. Ohshima, Y. Yokoyama, T. Takahashi, R. Shodo, K. Yamazaki, K. Ohtaki, T. Togashi, and Y. Sato. Drafting of the manuscript: R. Tanaka, Y. Ueki, and A. Horii. Final approval of the version to be published: All authors. Y. Ueki has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
This trial was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent before enrolling in the study. This study was approved by the Institutional Review Board of Niigata University Hospital (No. 2022-0157).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Tanaka, R., Ueki, Y., Ohshima, S. et al. Safety and efficacy of neoadjuvant chemotherapy with paclitaxel, carboplatin, and cetuximab for locally advanced head and neck squamous cell carcinoma. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02545-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02545-9