Abstract
Introduction
The timing of prophylactic pegylated granulocyte colony-stimulating factor (G-CSF) administration during cancer chemotherapy varies, with Day 2 and Days 3–5 being the most common schedules. Optimal timing remains uncertain, affecting efficacy and adverse events. This systematic review sought to evaluate the available evidence on the timing of prophylactic pegylated G-CSF administration.
Methods
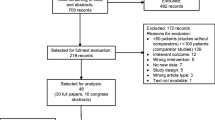
Based on the Minds Handbook for Clinical Practice Guideline Development, we searched the PubMed, Ichushi-Web, and Cochrane Library databases for literature published from January 1990 to December 2019. The inclusion criteria included studies among the adult population using pegfilgrastim. The search strategy focused on timing-related keywords. Two reviewers independently extracted and assessed the data.
Results
Among 300 initial search results, only four articles met the inclusion criteria. A meta-analysis for febrile neutropenia incidence suggested a potential higher incidence when pegylated G-CSF was administered on Days 3–5 than on Day 2 (odds ratio: 1.27, 95% CI 0.66–2.46, p = 0.47), with a moderate certainty of evidence. No significant difference in overall survival or mortality due to infections was observed. The trend of severe adverse events was lower on Days 3–5, without statistical significance (odds ratio: 0.72, 95% CI 0.14–3.67, p = 0.69) and with a moderate certainty of evidence. Data on pain were inconclusive.
Conclusions
Both Day 2 and Days 3–5 were weakly recommended for pegylated G-CSF administration post-chemotherapy in patients with cancer. The limited evidence highlights the need for further research to refine recommendations.




Similar content being viewed by others
Data availability
Data associated with this systematic review may be accessed from the corresponding author upon reasonable request.
References
Morizane T YM, Kojimahara N et al (2014) Minds Handbook for Clinical Practice Guideline Development 2014. Japan Council for Quality Health Care, Tokyo
Kojimahara N NT, Morizane T et al (2017) Minds Manual for Guideline Development 2017. Japan Council for Quality Health Care, Tokyo
Loibl S, Mueller V, von Minckwitz G et al (2011) Comparison of pegfilgrastim on day 2 vs. day 4 as primary prophylaxis of intense dose-dense chemotherapy in patients with node-positive primary breast cancer within the prospective, multi-center GAIN study: (GBG 33). Support Care Cancer 19(11):1789–1795
Kahl C, Sayer HG, Hinke A et al (2012) Early versus late administration of pegfilgrastim after high-dose chemotherapy and autologous hematopoietic stem cell transplantation. J Cancer Res Clin Oncol 138(3):513–517
Lambertini M, Bruzzi P, Poggio F et al (2016) Pegfilgrastim administration after 24 or 72 or 96 h to allow dose-dense anthracycline- and taxane-based chemotherapy in breast cancer patients: a single-center experience within the GIM2 randomized phase III trial. Support Care Cancer 24(3):1285–1294
Zwick C, Hartmann F, Zeynalova S et al (2011) Randomized comparison of pegfilgrastim day 4 versus day 2 for the prevention of chemotherapy-induced leukocytopenia. Ann Oncol 22(8):1872–1877
Acknowledgements
The authors are grateful to Ms. Natsuki Narita for her contribution to the initial literature search. The authors would also like to thank Ms. Natsuki Fukuda for their valuable comments and suggestions.
Funding
The present study did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the present study. The first draft of the manuscript was written by YO All authors commented on the previous version of the manuscript. All authors read and gave their final approval.
Corresponding author
Ethics declarations
Conflicts of interests
Y.O. received honoraria from Daiichi-Sankyo, Pfizer, Chugai, Lilly, and Kyowa Kirin. TE.Y. received honoraria from Kyowa Kirin, Pfizer, Chugai, Eli Lilly, MSD, AstraZeneca, and Eisai. K.T. received honoraria from Ono, Chugai, Taiho, and Novartis. E.I. received honoraria from Eli Lilly, and research funding from MSD, Ono, Janssen Pharma and Takeda. Y.M. received honoraria from Ono, MSD, Takeda, Eisai, and Bristol Myers Squibb, and research funding from MSD and Ono. S.Y. received research funding from Otsuka. D.M. received honoraria from Janssen, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai, Zenyaku, MSD, SymBio, Sanofi, AbbVie, Takeda, Astra Zeneca and Bristol Myers Squibb, and research funding from Biopharma, Novartis, Kyowa Kirin, Ono, Chugai, Janssen, Takeda, Otsuka, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho, and Astra Zeneca. T.M. received honoraria from Astra Zeneca, Chugai, and Myriad Genetics. E.B. received honoraria from Chugai, and Daiichi-Sankyo, and research funding from Taiho and Chugai. T.KU. received honoraria from Chugai. T.KI. received honoraria from Sanofi. S.N. received honoraria from Kyowa Kirin. A.S. received honoraria and scholarship donation from Chugai and Taiho. T.T. received honoraria from Daiichi-Sankyo, Chugai, and Eli Lilly. Other authors declare that there are no conflicts of interest associated with this manuscript. The other authors have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Formal consent was not required for this type of study.
Consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ozaki, Y., Yokoe, T., Yoshinami, T. et al. Optimal timing of prophylactic pegylated G-CSF after chemotherapy administration for patients with cancer: a systematic review and meta-analysis from Clinical Practice Guidelines for the use of G-CSF 2022. Int J Clin Oncol 29, 551–558 (2024). https://doi.org/10.1007/s10147-024-02499-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-024-02499-y