Abstract
Background
To evaluate the feasibility of the use and continuation of sentinel lymph node navigation surgery (SNNS) as an alternative to pelvic lymph node dissection (PLND) for patients with preoperatively estimated stage IA endometrial cancer.
Methods
This retrospective study selected the electronic medical records of all patients who had received CT scans and MRI imaging before surgery from April 1, 2009 to March 31, 2021. Sentinel lymph nodes (SLNs) were detected by administrating 99mTc-phytate and/or indocyanine green into the cervix, and the clinical outcomes of the patients who underwent SNNS or PLND were evaluated. Furthermore, in case of nodal recurrence, a new procedure to determine whether the facility should continue with SNNS or not was developed that compares the maximum likelihood hypothesis and an alternative one based on recurrence rates.
Results
Among 137 patients, SLN biopsies with ultrastaging were performed on 91 patients. The SLN detection rate was 95.6%. Over a 59-month median observation period, no statistically significant differences were shown in overall survival, disease-specific survival and disease-free survival between the SNNS and PLND groups when introducing the propensity score method (p-values: 0.06, 0.153, and 0.625, respectively). Our procedure demonstrated that, in our department without recurrence up to the 65th attempt, it was possible to continue SNNS if a recurrence occurs at the 66th attempt.
Conclusion
This study suggests the validity of SNNS as an alternative to PLND. Even in the absence of evidence from randomized controlled trials, we can confirm the validity of continuing SNNS using our procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer is the most common gynecologic malignancy in Japan. In 2018, among patients numbering more than 17,000, more than half were diagnosed with early-stage cancer not requiring additional treatments [1]. However, stage IIIC, as diagnosed by pelvic lymph node dissection (PLND), is speculated to have a relatively poor prognosis. For this reason, comprehensive staging surgery including PLND plays an important role in determining postoperative adjuvant therapy. The incidence of PLN metastasis in patients with low risk of recurrence confirmed pathologically is approximately 1.7% to 13% [2, 3]. Since the therapeutic impact of PLND on early-stage patients has yet to be clarified [4,5,6], it remains controversial whether PLND is necessary for patients with preoperatively estimated stage IA.
Since 2011, sentinel lymph node biopsy (SLNB) for early-stage patients has been used as a diagnostic method for assessing PLN status [7]. Thus, sentinel lymph node navigation surgery (SNNS), in which only one or a few SLNs are removed and further PLND is omitted when metastasis is ruled out intraoperatively, has been suggested as a way to reduce intraoperative surgical damage and postoperative complications such as leg edema and lymph cysts without worsening prognosis as long as certain criteria are met [8,9,10]. Furthermore, from 2013 to 2014, it was reported that identifying micrometastasis by ultrastaging and offering the opportunity for additional treatment to patients may improve survival compared with that of patients who underwent PLND without ultrastaging [11, 12].
At present, most reports on SNNS, which is a procedure that still does not have national health insurance coverage in Japan, focus on the surgical outcomes of SLNB and the reduction of leg edema and lymph cysts [13,14,15,16,17,18,19,20,21]. Only one report highlights oncologic outcomes, incorporating three-year overall survival and recurrence-free survival exclusively for the SNNS group [22]. Thus, data comparing the long-term outcomes of SNNS with those of PLND are lacking.
Furthermore, it is essential to maintain high metastatic identification and survival rates when introducing SNNS as an alternative to PLND at each facility. However, caution should be exercised when performing SNNS on patients indicated for PLND in standard clinical practice, as the eligibility and exclusion criteria for randomized controlled trials (RCTs) evaluating SNNS have not yet been reported [23, 24]. Given the low recurrence rate in early-stage patients, there is a risk of continuing SNNS after a recurrence without recognizing the surgical and diagnostic issues.
In this study, we assessed whether SNNS serves as an alternative to PLND by comparing the oncologic outcomes of the SLNB and PLND groups in patients with a preoperatively estimated stage IA. Furthermore, we report a new procedure that compares two different hypotheses to determine whether SNNS should continue if a recurrence occurs before a sufficiently large number of SNNS procedures have been performed [25].
Patients and methods
Patients
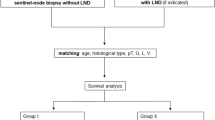
A consecutive series of patients with endometrial cancer who visited our facility between April 1, 2009, and March 31, 2021, were identified from the hospital`s electronic medical records. All patients were pathologically diagnosed with endometrial cancer, and patients who were preoperatively estimated with stage IA based on preoperative CT scans and MRI imaging were selected for the study. The preoperatively estimated stage IA group was divided into two groups, SLNB or PLND, excluding cases where neither SLNB nor PLND was performed (Fig. 1). The SNNS group was composed of patients who chose to omit further PLND when negative metastasis in SLN was detected intraoperatively and the PLND group was composed of patients who underwent bilateral PLND regardless of whether SLNB was performed.
Surgical procedures, pathological diagnosis
SLNB procedures for early-stage uterine malignancies have been performed with the approval of our hospital's Institutional Review Board since November 2010, while SNNS procedures began on December 1, 2012, when ultrastaging was introduced. SLNB and SNNS are not standard methods for assessing lymph node metastasis in Japan; patients themselves must make the decision to undergo PLND or SLNB after receiving an explanation of the procedures for those surgeries. If SLNB is chosen, the patient decides whether to undergo SNNS or PLND regardless of the results of the intraoperative diagnosis.
On the day before the surgery, 99mTc-phytate (PDR Pharma, Japan) was injected into the cervix at the 3 o'clock and 9 o'clock positions at a dose of 40 MBq/0.4 ml. Then, lymphoscintigraphy was performed 2 h later to estimate the location of the SLN. On the day of the surgery, 1 ml of indocyanine green (ICG) 0.025 mg/ml (Diagnogreen, Daiichi Sankyo, Japan) was administrated at the 2, 4, 8 and 10 o'clock positions of the cervix, respectively. The SLN was detected using a gamma probe (Navigator GPS, Sheeman Co. Ltd., Japan) and a fluorescent camera (Visera Elite II video system, Olympus, Japan); then it was excised and sent to a pathologist for intraoperative frozen section diagnosis [26, 27]. The diagnostic algorithm of SNNS has as a side-specific PLND: when there is an intraoperative diagnosis of SLN negativity, further removal of PLNs is omitted, and PLND is performed on the positive SLN side or on the side where there is an undetected SLN [8, 9]. Para-aortic lymph node (PAN) dissection was performed with PLND when the patient was preoperatively diagnosed as high-risk and gave consent for PAN dissection, regardless of whether SLNB was performed. An extrafascial extended hysterectomy, a standard hysterectomy in Japan, was performed. Intraoperative diagnosis of cancer metastasis was made by taking frozen section specimens at 2-mm intervals, which were perpendicularly sliced and stained with H&E. After preparing formalin-fixed paraffin embedded tissue (FFPE), the remaining specimens were ultrastaged. Immunohistochemical analysis using cytokeratin AE1/AE3 was performed at 20-μm intervals on a 3-μm FFPE section to detect micrometastasis. A routine histopathological examination was made to evaluate the FIGO stage and risk of recurrence. Low risk was defined as FIGO stage IA endometrioid carcinoma grade 1 or grade 2 with negative lympho-vascular space invasion (LVSI). Intermediate and high risk were defined as any risk other than low risk. For patients postoperatively diagnosed as intermediate or high risk, an additional six cycles of chemotherapy or radiation therapy, and three cycles of chemotherapy if isolated tumor cells (ITC) were confirmed, were proposed and those additional treatments were performed unless refused.
Statistical analysis
The data obtained were summarized using basic statistics and tested using either a Mann–Whitney test, a chi-square test, or Fisher's exact test. The propensity score method (pair-matching) was utilized to.
adjust for confounding factors in the SNNS and PLND groups. Age (under or over 55), LVSI (negative or positive), histologic subtype (either endometrioid carcinoma grade 1 or 2, or others) and upstaging (FIGO stage IA or others) were used as explanatory variables [28,29,30]. The propensity score was calculated by logistic regression analysis. The matching caliper was set to 0.2 and it was used to create a 1:1 matched pair from both groups. Then, the survival rates of the created groups were compared using the adjusted Kaplan–Meier curves with a log-rank test. The results were considered significant at p < 0.05 and all tests were two-tailed. All survival analyses were performed using EZR 1.52 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a GUI of R ver.4.0.0 (The R Foundation for Statistical Computing, Vienna, Austria) [31].
The start of observation was the date of surgery, and the end of observation was either the last hospital visit or death confirmation by September 30, 2021. Overall survival (OS) was calculated from all-cause death events, while disease-specific survival (DSS) was calculated from death events due to recurrence diagnosed by CT scan every six months or from vaginal stump cytology. Disease-free survival (DFS) is defined as the period from the start date to the earlier of either the date of recurrence or the date of death from any cause.
Development of evaluation procedure for judging whether to continue with SNNS
The nodal recurrence rate after SNNS, P(A), is 1.2% and the nodal recurrence rate after PLND, P(B), is 1.7% in a systematic review [32]. Consider a facility H where PH(A), the true recurrence rate after SNNS, is 1.2%. It is obvious that SNNS should be performed if they know the value of PH(A). However, they have no way of knowing it. The only performance index that they can use to judge whether to continue SNNS or not is thus PH(A|N), the recurrence rate obtained from N SNNSs (the number of SNNSs) performed at the facility H. However, in the case of PH(A) = 1.2% and P(B) = 1.7%, P(PH(A|N) > P(B)), the probability that PH(A|N) is greater than P(B), is 50.4% when N = 58 if recurrences occur randomly with probability PH(A). Therefore, it is difficult for the facility to make a judgement on the basis of PH(A|N) about whether they should continue performing SNNS if PH(A|N) > P(B) at the beginning of SNNS. We thus developed a new procedure to solve this problem when PH(A|N) > P(B), as shown in Fig. 2. Here, K is a sufficiently large value greater than one: K = 2 in this study. Note that it is obvious that SNNS should be continued regardless of the value of \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)), an estimate of P(PH(A|N) > P(B)), when PH(A|N) ≦ P(B).
Consider an example where N = 20 and nA = 1 (PH(A|N) = 5.0%, Fig. 3a). According to the procedure described above, SNNS should be continued in this case, although PH(A|N) = 5.0% is sufficiently greater than P(B) = 1.7%. This is because \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)) for the maximum likelihood hypothesis is 85.0% and is less than two times that for the alternative hypothesis (61.7%) (Table 1); that is, PH(A) > P(B) is insignificant (the alternative hypothesis is significant) according to the procedure with K = 2. However, if nA = 3 for N = 60 (see Fig. 3b), SNNS should be discontinued although PH(A|N) = 5.0%, in the same manner as in the above case with N = 20. This is because \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)) for the maximum likelihood hypothesis is 89.9% and is greater than two times that for the alternative (43.8%) (Table 1); that is, PH(A) > P(B) is significant (the maximum likelihood hypothesis is significant). We can thus evaluate the validity of performing SNNSs in an actual situation where N is less than 100 by using a procedure with a criterion that is intuitively easy to understand.
\(\tilde{{\text{P}}}\)(PH(A|N) > P(B)) [%] (P(B) = 1.7% for maximum likelihood hypotheses and alternative hypothesis. a: at 1 event per 20 attempts, b: at 3 events per 60 attempts. P(B): Recurrence rate after PLND (1.7%) obtained from a systematic review. PH(A): True recurrence rate after SNNS in facility H, which it is impossible to know in advance. nA: The number of recurrences in N SNNSs (a random variable). ◆: A binomial distribution B (N, PH (A)) that obeys nA. PH(A|N) = nA/N: Recurrence rate after SNNS obtained from N SNNSs performed in facility H, which is a random variable because nA is a random variable. ─◆─: Probability density function for PH(A|N), which is an approximation of B (N, PH (A)) when PH (A|N) is treated as a continuous random variable. *: Area under curve (AUC) based on the probability density function of each hypothesis. For instance, 61.7 for the alternative hypothesis in 20 attempts means the percentage to the right of 0.017 when the overall area of the graph is set to 1. \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)): The areas indicated by * in the four graphs, which means an estimate of the probability that PH (A|N) is greater than P(B)
Results
Surgical and pathological outcomes
A total of 218 consecutive pathologically confirmed patients were selected from the hospital`s electronic medical records, and 91 out of the 137 patients with preoperatively estimated stage IA underwent SNLB with ultrastaging. The patients’ characteristics and clinical results are shown in Table 2. Of those with preoperative estimated stage IA, 76.9% were FIGO stage IA, 23.1% were upstaged to stage IB or higher, and the rate of the histologic type of endometrioid carcinoma grade 1 or 2 was 82.4%. The overall detection rate and bilateral detection rate of SLN were 95.6% and 78.0%, respectively. The detection rates with 99mTc-phytate and/or ICG were 90% or more and there was no difference in detection rate between two tracers (p = 0.57). Metastasis and ITC to SLN were identified in 9.9% and 6.6% of the patients, respectively. No surgical complications were found to have occurred during SNLB.
Survival analysis
Of the 137 patients, 65 underwent SNNS and 42 underwent PLND. The median observation time for the 107 patients in the SNNS and PLND groups was 59 months. The results of the survival analysis are shown in Fig. 4 and Table 3. There were significant differences in pathological type and LVSI ratio between the two groups (p = 0.01 and p = 0.026, respectively), so the propensity score method was used to adjust for confounding factors. As a result, log-rank tests of adjusted OS, DSS, and DFS showed p-values of 0.06, 0.153 and 0.625, respectively, indicating no significant difference between the two groups. The five-year survival rates were 100% and 85.5% for SNNS and PLND, respectively, and the five-year DSS rates were 100% and 93.6%.
Evaluation of procedure for judging whether to continue with SNNS
We discussed the advisability of continuing SNNS attempts in our department, which has had 65 SNNS attempts, by using the procedure developed in this study. As a result, it was found that it is possible to continue SNNS if a recurrence occurs at the 66th attempt. This is because there was no recurrence up to the 65th attempt in our department and thus \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)) was less than the given criterion (Table 1).
Discussion
In 2008, Marian et al. proposed an option to omit PLND for early-stage endometrial cancer patients with a low risk of recurrence under certain conditions. However, this approach had an issue of low specificity despite its high sensitivity in detecting PLN metastasis [33, 34]. When SNNS is introduced in each facility as an alternative to PLND, it is important to maintain metastatic identification and survival rates that are at least comparable to those of PLND while ensuring a high detection rate for SLN. So far, methods using ICG and a fluorescence camera have been reported to achieve sufficiently high SLN detection rates [35, 36]. Moreover, in seven recent studies using 99mTc, the detection rates ranged from 91–98% (median 93.8%) overall, and 63–88% (median 79.2%) for bilateral findings [19, 37–42]. Additionally, a significant amount of data from the Japan Society of Gynecology and Obstetrics indicates the five-year survival rate for stage IA patients in Japan at around 96% [1]. If these conditions are met, SNNS could potentially replace PLND as a less invasive procedure.
This is the first report on oncologic outcomes, such as OS and DSS, for the SNNS and PLND groups of Japanese patients with a preoperatively estimated stage IA endometrial cancer. In this study, the detection rates of SLN and SLN metastases were comparable to previous reports, and there was no statistically significant difference in adjusted OS using the propensity score method between the SNNS and PLND groups. Furthermore, DSS and DFS were calculated as additional indicators for clinical judgment, and it was concluded that there was no significant difference in any of the survival outcomes between the two groups. Additionally, the five-year survival rate of patients who underwent SNNS at our institution appeared to align with data from a large Japanese database. Although SNNS in this study seems to be a promising alternative diagnostic approach for detecting PLN metastasis and reducing surgical damage, we must ultimately await the results of reliable RCTs with a larger sample size to determine if SNNS is a suitable diagnostic alternative to PLND.
Lymph node recurrence rates, which may be related to survival rates, can also serve as an indicator of the validity of SNNS. Generally, due to the learning curve, surgeons typically require 30 to 40 procedures to perform SNNS reliably [43,44,45]. When a nodal recurrence is significantly more frequent with SNNS than with PLND, SNNS should be discontinued. However, for instance, if a nodal recurrence occurs in fewer than 100 attempts, especially at the beginning of SNNS, it might be difficult to distinguish between a random error, where recurrence might occur even with PLND, and a systematic error from SNNS surgical technique. To solve this problem, we need a statistical test to judge whether SNNS should be continued or not. Also, we may need a statistical matching technique such as propensity score matching to reduce the bias due to the effect of a treatment or other intervention. However, we cannot always use these conventional techniques. This is because P(A) and P(B) are too small (1.2% and 1.7%) for the above situation where a sufficiently large number of SNNSs have not been performed at each facility at the beginning of SNNS, and the conventional techniques mentioned above need a sufficiently large number of attempts to evaluate the results of SNNS with high accuracy. We thus developed a new procedure that involves comparing the maximum likelihood hypothesis, where PH(A) = PH(A|N), with the alternative, where PH(A) = 1.2%. The procedure can be used at the beginning of SNNS when there have been fewer than 100 attempts and it shows us whether PH(A|N) is within an allowable dispersion or not. We can thus stop performing SNNS if PH(A|N) is beyond the allowable dispersion and can investigate the reason why it is high. This enables us to decrease the chances that patients suffer from selecting the wrong operative method. As mentioned before, PH(A|N) is a random variable; \(\tilde{{\text{P}}}\)(PH(A|N) > P(B)) thus contains a stochastic dispersion of up to two times when recurrences occur randomly. Therefore, we set K = 2 in our study. However, recurrences do not always occur randomly. Thus, it is necessary to investigate the recurrence course and establish a method to set the value of K.
In conclusion, SNNS with cervical injection of tracers appears to be a safe alternative to PLND without increasing recurrences or decreasing survival rates, provided the SLN detection rate remains high and micrometastasis is identified through ultrastaging. However, as evaluations of SNNS using RCTs have not yet been reported, caution should be exercised in continuing SNNS, especially in the event of a nodal recurrence. The decision to proceed with SNNS should be based on verifiable and appropriate criteria. In order for SNNS to be covered by national health insurance in Japan, it is necessary to increase the number of cases or the number of facilities performing SNNS and to demonstrate its efficacy as an alternative to PLND. Additionally, the issues due to financial or human resources, such as the adoption of OSNA (one-step nucleic acid amplification) assays and pathological ultrastaging methods for detecting SLN metastasis, remain to be addressed in the future.
References
Japanese Society of Obstetrics and Gynecology. Gynecologic Oncology Committee Report 2020. Available from: http://fa.kyorin.co.jp/jsog/readPDF.php?file=72/7/072070800.pdf
Creasman WT, Morrow CP, Bundy BN et al (1987) Surgical pathologic spread patterns of endometrial cancer. A Gynecol Oncol Group Study Cancer 60:2035–2041
Pölcher M, Rottmann M, Brugger S et al (2019) Lymph node dissection in endometrial cancer and clinical outcome: a population-based study in 5546 patients. Gynecol Oncol 154:65–71
Benedetti Panici P, Basile S, Maneschi F et al (2008) Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst 100:1707–1716
Kitchener H, Swart AM, Qian Q et al (2009) Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet 373:125–136
Frost JA, Webster KE, Bryant A et al (2017) Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007585.pub4
Ballester M, Dubernard G, Lécuru F et al (2011) Detection rate and diagnostic accuracy of sentinel-node biopsy in early-stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol 12:469–476
Cormier B, Diaz JP, Shih K et al (2011) Establishing a sentinel lymph node mapping algorithm for the treatment of early cervical cancer. Gynecol Oncol 122:275–280
Barlin JN, Khoury-Collado F, Kim CH et al (2012) The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol 125:531–535
Helgers RJA, Winkens B, Slangen BFM et al (2020) (2020) Lymphedema and post-operative complications after sentinel lymph node biopsy versus lymphadenectomy in endometrial carcinomas-a systematic review and meta-analysis. J Clin Med 10:120
Kim CH, Khoury-Collado F, Barber EL et al (2013) Sentinel lymph node mapping with pathologic ultrastaging: a valuable tool for assessing nodal metastasis in low-grade endometrial cancer with superficial myoinvasion. Gynecol Oncol 13:714–719
Raimond E, Ballester M, Hudry D et al (2014) Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol Oncol 133:506–511
Yamagami W, Susumu N, Kataoka F et al (2017) A comparison of dye versus fluorescence methods for sentinel lymph node mapping in endometrial cancer. Int J Gynecol Cancer 27:1517–1524
Tanaka T, Terai Y, Fujiwara S et al (2018) The detection of sentinel lymph nodes in laparoscopic surgery can eliminate systemic lymphadenectomy for patients with early stage endometrial cancer. Int J Clin Oncol 23:305–313
Shimada C, Todo Y, Yamazaki H et al (2018) A feasibility study of sentinel lymph node mapping by cervical injection of a tracer in Japanese women with early stage endometrial cancer. Taiwan J Obstet Gynecol 57:541–545
Togami S, Kubo R, Kawamura T et al (2020) Risk factors for lymphatic complications following lymphadenectomy in patients with endometrial cancer. Taiwanese J Obstet Gynecol 59:420–424
Niikura H, Toki A, Nagai A et al (2021) Prospective evaluation of sentinel node navigation surgery in Japanese patients with low-risk endometrial cancer-safety and occurrence of lymphedema. Jpn J Clin Oncol 51:584–589
Togami S, Ushiwaka T, Kitazono I et al (2022) One-step nucleic acid amplification (OSNA) assay for detecting lymph node metastasis in cervical and endometrial cancer: a preliminary study. J Gynecol Oncol 33:e11
Togami S, Ushiwaka T, Fukuda M et al (2022) Comparison of radio-isotope method with 99m technetium and near-infrared fluorescent imaging with indocyanine green for sentinel lymph node detection in endometrial cancer. Jpn J Clin Oncol 52:24–28
Terada S, Tanaka T, Murakami H et al (2023) Lymphatic complications following sentinel node biopsy or pelvic lymphadenectomy for endometrial cancer. J Clin Med 12:4540
Togami S, Tanimoto A, Yanazume, et al (2023) Evaluation of the one-step nucleic acid amplification assay for detecting lymph node metastasis in patients with cervical and endometrial cancer: a multicenter prospective study. Gynecol Oncol 70:70–76
Togami S, Fukuda M, Mizuno M et al (2023) Efficacy and prognosis of robotic surgery with sentinel node navigation surgery in endometrial cancer. J Gynecol Oncol 34:e68
Park JY, Kim JH, Baek MH et al (2022) Randomized comparison between sentinel lymph node mapping using indocyanine green plus a fluorescent camera versus lymph node dissection in clinical stage I-II endometrial cancer: a Korean gynecologic oncology group trial (KGOG2029/SELYE). J Gynecol Oncol 33:e73
Baiocchi G, Andrade CEMC, Ribeiro R et al (2022) Sentinel lymph node mapping versus sentinel lymph node mapping with systematic lymphadenectomy in endometrial cancer: an open-label, non-inferiority, randomized trial (ALICE trial). Int J Gynecol Cancer 32:676–679
Yamashita T, Ito T, Akimoto T, et al (2021) Feasibility and validity of sentinel node navigation surgery in patients with stage IA endometrial cancer (in Japanese). Nihon Sanka Fujinka Naishikyou Gakkai zasshi. 37:76-83
Yamashita T, Katayama H, Kato Y et al (2009) Management of pelvic lymph nodes by sentinel node navigation surgery in the treatment of invasive cervical cancer. Int J Gynecol Cancer 19:1113–1118
Plante M, Touhami O, Trinh XB et al (2015) Sentinel node mapping with indocyanine green and endoscopic near-infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature. Gynecol Oncol 137:443–447
Cianci S, Rosati A, Vargiu V et al (2021) Sentinel lymph node in aged endometrial cancer patients “the sage study”: a multicenter experience. Front Oncol 11:737096
Ghoniem K, Larish AM, Dinoi G et al (2021) Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: An international multi-institutional study. Gynecol Oncol 162:590–598
Matanes E, Eisenberg N, Lau S et al (2021) Absence of prognostic value of lymphovascular space invasion in patients with endometrial cancer and negative sentinel lymph nodes. Gynecol Oncol 162:256–261
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458
Bogani G, Murgia F, Ditto A et al (2019) Sentinel node mapping vs. lymphadenectomy in endometrial cancer: a systematic review and meta-analysis. Gynecol Oncol 153:676–683
Mariani A, Dowdy SC, Cliby WA et al (2008) Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol 109:11–18
Lefringhouse JR, Elder JW, Baldwin LA et al (2017) Prospective validation of an intraoperative algorithm to guide surgical staging in early endometrial cancer. Gynecol Oncol 145:50–54
Rossi EC, Kowalski LD, Scalici J et al (2017) A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol 18:384–392
Frumovitz M, Plante M, Lee PS et al (2018) Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol 19:1394–1403
Restaino S, Buda A, Puppo A et al (2022) Anatomical distribution of sentinel lymph nodes in patients with endometrial cancer: a multicenter study. Int J Gynecol Cancer 32:517–524
Tal O, Grinstein E, Goshen E et al (2021) Anatomic asymmetry in sentinel lymph node detection in endometrial cancer. J Minim Invasive Gynecol 28:1531–1535
Jayot A, Owen C, Bendifallah S et al (2021) Relevance of sentinel lymph node biopsy in early endometrial cancer: a series of 249 cases. Eur J Obstet Gynecol Reprod Biol 258:208–215
Cabrera S, Barahona-Orpinell M, Almansa-González C et al (2021) Combined use of ICG and technetium does not improve sentinel lymph node detection in endometrial cancer: results of the COMBITEC study. Gynecol Oncol 162:32–37
Martinelli F, Ditto A, Bogani G et al (2020) Sentinel lymph node mapping in endometrial cancer: performance of hysteroscopic injection of tracers. Int J Gynecol Cancer 30:332–338
Kessous R, How J, Abitbol J et al (2019) Triple tracer (blue dye, indocyanine green, and Tc99) compared to double tracer (indocyanine green and Tc99) for sentinel lymph node detection in endometrial cancer: a prospective study with random assignment. Int J Gynecol Cancer 29:1121–1125
Khoury-Collado F, Glaser GE, Zivanovic O et al (2009) Improving sentinel lymph node detection rates in endometrial cancer: how many cases are needed? Gynecol Oncol 115:453–455
Thomaier L, Jager L, Stone R et al (2019) Risk of empty lymph node packets in sentinel lymph node mapping for endometrial cancer using indocyanine green. Int J Gynecol Cancer 29:513–517
Tucker K, Staley SA, Gehrig PA et al (2020) Defining the learning curve for successful staging with sentinel lymph node biopsy for endometrial cancer among surgeons at an academic institution. Int J Gynecol Cancer 30:346–351
Acknowledgements
The authors would like to thank all of our colleagues helping with surgical treatments in our department.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The study followed the Declaration of Helsinki and received approval from Hakodate Municipal Hospital’s Institutional Review Board (IRB# 2022-050)
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yamashita, T., Itoh, T., Asano, T. et al. Clinical outcomes of sentinel node navigation surgery in patients with preoperatively estimated stage IA endometrial cancer and evaluation of validity for continuing sentinel node navigation surgery based on dispersion of recurrence probability. Int J Clin Oncol 29, 222–231 (2024). https://doi.org/10.1007/s10147-023-02449-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02449-0