Abstract
Background
Enteral feeding (EF) is recommended to enhance nutritional status after esophagectomy; however, diarrhea is a common complication of EF. We investigated the clinical and prognostic impact of diarrhea during EF after esophagectomy.
Methods
One hundred and fifty-two patients who underwent transthoracic esophagectomy were enrolled. The King's stool chart was used for stool characterization. The short- and long-term outcomes were compared between a non-diarrhea (Group N) and diarrhea group (Group D).
Results
A higher dysphagia score (≥ 1) was observed more frequently in Group D than in Group N (45.7% vs. 19.8%, p = 0.002). Deterioration of serum total protein, serum albumin, serum cholinesterase, and the prognostic nutritional index after esophagectomy was greater in Group D than in Group N (p = 0.003, 0.004, 0.014, and 0.001, respectively). Patients in Group D had significantly worse overall survival (OS) and recurrence-free survival (RFS) than those in Group N (median survival time (MST): OS, 21.9 vs. 30.6 months, p = 0.001; RFS, 12.4 vs. 27.7 months, p < 0.001). In stratified analysis due to age, although there was no difference in OS with or without diarrhea in young patients (MST: 24.1 months in a diarrhea group vs. 33.6 months in a non-diarrhea group, p = 0.218), patients in a diarrhea group had significantly worse OS than those in a non-diarrhea group in elderly patients (MST: 17.8 months vs. 27.9 months, p < 0.001).
Conclusions
Diarrhea during EF can put elderly patients at risk of postoperative malnutrition and a poor prognosis after esophagectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global cancer statistics show that esophageal cancer is the sixth leading cause of cancer-related deaths worldwide [1]. Patients with esophageal cancer have many nutritional risks. Masses often cause solid food obstruction preoperatively, and oral intake must be ceased for a certain period postoperatively to protect the anastomosis site [2]. Additionally, transthoracic esophagectomy, recognized as a principal treatment for esophageal cancer [3, 4], is more invasive, resulting in a systemic inflammatory response and poses a risk for postoperative complications [5]. We previously reported that enhancing postoperative nutritional status in patients with preoperative malnutrition leads to a better prognosis after esophagectomy [6]. Other studies have suggested that nutritional management in the early postoperative period is important to enhance cell-mediated immunity after esophagectomy [5, 7, 8]. Enteral feeding (EF) has been recommended as supportive therapy for patients after surgery or in intensive care [2, 5, 7, 9]. EF has been shown to promote nitrogen retention, restore immune function, and accelerate wound healing [10]. Postoperative EF has also been shown to reduce the risk of septic complications after esophagectomy [2].
However, the complications associated with specific EF methods may diminish the intended benefits. Diarrhea is a major complication of EF [10, 11]. The incidence of diarrhea during EF in intensive care is reported to be 2–95% [2, 10,11,12]. In addition, diarrhea has been linked to malnutrition [2, 10]. In intensive care, the combination of stress and malnutrition is associated with a negative energy balance, which leads to delayed wound healing, prolonged hospital stay, and higher healthcare costs [12]. Furthermore, malnutrition is associated with increased morbidity and mortality during critical illness [2, 12]. However, the clinical significance of diarrhea during EF after esophagectomy remains unknown.
This study hypothesizes that diarrhea during EF after esophagectomy could be a risk factor for postoperative malnutrition and poor prognosis. Therefore, we investigated the associations between diarrhea during EF with short- and long-term outcomes.
Patients and methods
Patients
Between January 2017 and December 2021, 178 patients with esophageal and esophagogastric junction cancer (EGJC) were retrospectively reviewed. All patients underwent esophagogastroduodenoscopy (EGD) and computed tomography (CT) from the neck to the pelvis to determine the clinical stage. The clinical and pathological stages were determined based on the Union for International Cancer Control TNM classification of malignant tumors, 8th edition [13].
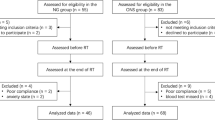
Patients who met the following criteria were enrolled in this study: (1) age > 20 years, (2) Eastern Cooperative Oncology Group performance status ≤ 1, (3) radical esophagectomy, (4) reconstruction via a gastric conduit, (5) no double cancer, (6) no prior irradiation, (7) one-stage surgery, and (8) follow-up for more than 1 year. Patients were excluded from the study based on the following criteria: salvage surgery (n = 8), colon conduit reconstruction (n = 10), invasion to surrounding organs (n = 2), residual disease (n = 1), postoperative in-hospital death (n = 1), and interruption of follow-up within 1 year (n = 4). Finally, 152 patients were included in this study (Fig. 1).
Multidisciplinary treatment
In accordance with the esophageal cancer practice guidelines 2017 in Japan, neoadjuvant chemotherapy (NAC) was administered to patients with non-Stage I squamous cell carcinoma (SCC) [3, 4]. At our institution, patients with adenocarcinoma with bulky lymph node (LN) metastases underwent NAC. For SCC, the treatment regimens were a combination of cisplatin and 5-fluorouracil or a combination of docetaxel, cisplatin, and 5-fluorouracil for SCC and a combination of S-1 and oxaliplatin for adenocarcinoma. A right transthoracic subtotal esophagectomy with 2- or 3-field LNs dissection was performed as a standard surgical procedure at our institution [14, 15]. Upper, middle, and lower mediastinal LNs and abdominal LNs were routinely dissected. The upper mediastinal region included the upper thoracic paraesophageal nodes, and left and right paratracheal nodes; the middle mediastinal region included the middle thoracic paraesophageal nodes, subcarinal nodes, and main bronchus nodes; and the lower mediastinal region included the lower thoracic paraesophageal nodes, posterior mediastinal nodes, and supradiaphragmatic nodes. In the abdominal region, bilateral paracardial nodes, lesser curvature nodes, and LNs along the left gastric artery, common hepatic artery, celiac artery, and proximal splenic artery were dissected. Except for patients with low surgical tolerance or high surgical risk, bilateral cervical LNs dissection was generally performed for advanced cancer or superficial cancer in the middle or upper thoracic esophagus. Gastric conduit reconstruction via the posterior mediastinal route was performed with hand-sewn anastomosis in the neck. The retrosternal route was selected when the risk of anastomotic leakage (AL) was considered high, such as in those who took steroids or suspected insufficient blood flow in the gastric conduit. In posterior mediastinal route reconstruction, a 12-Fr jejunostomy catheter was inserted into the proximal jejunum. Further, we inserted this into the gastric antrum in retrosternal route reconstruction. Following esophagectomy, cefazolin 1 g was administered twice daily via a peripheral intravenous line for 3 days as a prophylactic antibiotic.
The Clavien–Dindo classification was used to assess postoperative complications such as pneumonia, AL, and surgical site infection (SSI). Further, postoperative complications of grade ≥ II were identified [16]. Postoperative body weight (BW), body mass index (BMI), serum total protein, serum albumin, serum cholinesterase, prognostic nutritional index (PNI) were used to assess nutritional status [17]. Additionally, the serum C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR) were used as inflammatory markers. BW and BMI were measured before and at 1 and 3 months after surgery. Other parameters were measured in blood samples taken before and 1 month after surgery. These parameters were measured after NAC but before surgery in patients who received NAC. Patients were divided into high and low groups using median values for PNI and NLR and the institutional reference level for CRP (0.3 mg/dl).
Perioperative nutritional support
In April 2017, a multidisciplinary support team was established to prevent complications and improve nutritional status [18]. Before treatment, dietitians assessed the oral intake and recommended oral nutritional supplements for those whose calorie intake was insufficient. Patients with severe dysphagia caused by massive tumors were admitted early, and EF via a nasogastric tube was performed during NAC. Other patients were admitted 5 days prior to surgery and received oral nutritional support the following day. An elemental diet was started at 10 ml/h (240 kcal/day) on the day of surgery via a jejunostomy tube. EF speed was gradually increased to 50 ml/h (1200 kcal/day) based on the patient’s abdominal condition (Online Resource 1). EF was reduced or stopped temporarily because of diarrhea, abdominal fullness, chylothorax, or anorexia. On postoperative day 7, the EF agent was gradually changed from an elemental diet to a fat-containing agent. CT and upper gastrointestinal contrast imaging were performed on postoperative day 7 to ensure that there are no complications such as AL. Video-fluoroscopic and video-endoscopic examinations of the swallowing function were performed before starting oral intake (Online Resource 1). However, patients with AL were deferred from starting the oral diet. When diarrhea occurred, the EF speed was reduced first. We changed the EF agent or started probiotics if no improvement was observed. A multidisciplinary team conference was held to discuss the required calories, nutritional status, dietary intake, EF agents, and stool condition of the patients [6, 18]. EF was continued in patients after hospital discharge until oral intake was satisfactory. The degree of preoperative food passage obstruction was assessed using the dysphagia score (Table 1).
Assessment of fecal output
The King’s Stool Chart (KSC), which incorporates the frequency, consistency, and weight of fecal output during EF, was used to assess diarrhea (Table 2) [19]. The daily fecal score was calculated by scoring fecal conditions into 12 categories and summing the daily scores. Patients with KSC scored ≥ 16 should have their EF speed, or agents changed [19, 20]. In this study, diarrhea was defined as KSC ≥ 16 for 3 days.
Follow-up
For 5 years after surgery, CT was performed every 6 months, and EGD was performed yearly. Recurrence-free survival (RFS) was calculated from the day of surgery to the day of esophageal cancer or EGJC recurrence. Overall survival (OS) was calculated from the day of surgery to the day of death. Patients were followed up until death, five years after esophagectomy, or the study termination (December 31, 2022). Patients who were alive at the study termination, interrupted follow-up, and died due to an illness unrelated to their primary disease were recognized as censored.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 26 for Windows (IBM Corp., Armonk, NY, USA). Medians and ranges were calculated, and differences were identified using the Mann–Whitney U test. Differences between categorical variables were identified using the Chi-squared or Fisher’s exact test. Repeated measures analysis of variance was used to analyze the association between preoperative and postoperative nutritional status. Survival curves were generated using the Kaplan–Meier survival method and the log-rank test. Odds ratios (OR) and hazard ratios (HR) were calculated. Univariate and multivariate analyses were performed using logistic regression analysis for nominal variables and Cox proportional hazards regression models for survival analysis. Univariate and multivariate analyses of clinicopathologic factors that may be risk factors for diarrhea were performed using logistic regression analysis. The clinicopathological factors previously reported to be associated with prognosis, such as age, NAC, transthoracic approach, postoperative complications (pneumonia and AL), pathological stage, PNI, CRP, NLR, and diarrhea during EF, were evaluated by univariate and multivariate analysis using the Cox proportional hazards model. The threshold for significance was set at p < 0.05.
Results
Demographics and perioperative outcomes
Diarrhea occurred in 46 (30.3%) patients during EF, and patients were divided into a non-diarrhea (Group N, n = 106) and diarrhea group (Group D, n = 46) (Fig. 1). The clinicopathological features of each group are shown in Tables 3 and 4. This study defined elderly patients using a cut-off value of 70 years according to a previous study [21]. There was no significant difference in age, sex, histology, tumor location, clinical stage, or rate of NAC administration. Patients with a higher dysphagia score (≥ 1) were significantly found in Group D than in Group N (45.7% vs. 19.8%, p = 0.002) (Table 3). There were 19 (12.5%) patients who had reduced or stopped EF. Eleven (7.2%) patients had abdominal fullness and 8 (5.3%) patients had chylothorax. One of them was accompanied by abdominal fullness, chylothorax, and anorexia. However, all patients resumed EF after these symptoms improved. The morbidity of postoperative complications was similar between the two groups. In most patients, the oral diet was started on postoperative day 10 (median, range 7–35 days). The median duration from surgery to diarrhea onset was 10 days (range, 4–24 days), and it took several days after starting diarrhea management to achieve stool control (median 4 days, range 0–28 days). The duration of postoperative hospital stay was significantly longer in Group D compared to Group N (median 29 vs. 24 days, p = 0.011) (Table 4). Stool cultures were collected from 39 patients (28 in Group D and 11 in Group N), and revealed that Enterococcus spp. and Escherichia coli were frequently cultured in both groups. Furthermore, Clostridioides difficile and Klebsiella oxytoca, known to cause antibiotics-associated colitis, were found in a few patients in Group D (Online Resource 2).
Loss of postoperative nutritional status due to diarrhea
The impact of diarrhea on postoperative nutritional status was investigated (Fig. 2). There was no significant difference in BW (p = 0.325) and BMI (p = 0.526) decline between the two groups before and at 1 and 3 months after surgery (Fig. 2a, b). However, compared to Group N, postoperative serum total protein (p = 0.003), serum albumin (p = 0.004), serum cholinesterase (p = 0.014), and PNI (p = 0.001) were significantly decreased in Group D (Fig. 2c–f). In addition, NLR values in Group N decreased from the preoperative phase to 1 month after surgery, whereas NLR values in Group D increased during the same period (p = 0.042) (Fig. 2g). There were no significant differences in serum CRP levels between the two groups (p = 0.248) (Fig. 2g).
Next, the patients were divided into two subgroups: those with or without postoperative infectious complications (AL, pneumonia, and SSI). In the non-infectious complication group, PNI (45.1 vs. 41.0, p = 0.009) and CRP (0.18 mg/dl vs. 0.34 mg/dl, p = 0.001) levels at 1 month after surgery were significantly better than those in the infectious complication group. However, there were no significant differences in NLR values between the two groups (2.81 in the non-infectious complication group vs. 2.90 in the infectious complication group, p = 0.360) (Online Resource 3). Additionally, multivariate analysis identified that laparotomy and diarrhea during EF were relevant factors for postoperative high NLR values (Online Resource 4).
Risk factors for diarrhea during EF
Variables in Table 5 indicate potentially significant clinicopathological factors affecting diarrhea during EF. In multivariate analysis, a higher dysphagia score (≥ 1) (OR 3.781; p = 0.009; 95% confidence interval [CI] 1.398–10.227), NAC (OR 2.976; p = 0.040; 95% CI 1.050–8.436), and AL (OR 4.368; p = 0.022; 95% CI 1.232–15.488) were independent relevant factors for diarrhea during EF (Table 5). However, no correlation was observed between therapeutic antibiotic use and diarrhea (OR 1.349; p = 0.547; 95% CI 0.532–3.665).
Survival analysis
The median follow-up period was 27.5 months (range 1.9–66.3 months); 52 (34.2%) patients experienced recurrence. Patients in Group D had significantly higher rates of regional LN and distant organ recurrence than those in Group N (regional LN recurrence: 39.1% vs. 15.2%, p = 0.003; distant organ recurrence: 34.8% vs. 14.3%, p = 0.008) (Table 4). The rate of patients dying from illnesses unrelated to primary disease was similar in both groups (Group D 2.2% vs. Group N 4.7%, p = 0.668).
Patients in Group D had significantly worse OS and RFS than those in Group N (median survival time (MST) OS: 21.9 vs. 30.6 months, p = 0.001; RFS: 12.4 vs. 27.7 months, p < 0.001) (Fig. 3a, b). In addition, an advanced pathological stage (≥ III) (HR 4.172; p = 0.001; 95% CI 1.749–9.947) and diarrhea during EF (HR 2.174; p = 0.040; 95% CI 1.038–4.456) were independent predictors of poor OS in the multivariate analysis (Table 6).
Kaplan–Meier analysis based on the incidence of diarrhea during enteral feedings. a Comparison of overall survival between Groups N and D. b Comparison of recurrence-free survival between Groups N and D. c Comparison of overall survival between the younger groups (Groups N-Y and D-Y). d Comparison of recurrence-free survival between the younger groups (Groups N-Y and D-Y). e Comparison of overall survival between the elderly groups (Groups N-E and D-E). f Comparison of recurrence-free survival between the elderly groups (Groups N-E and D-E)
Next, the survival impact of the postoperative nutritional and immunological status (CRP, PNI, and NLR) was investigated. Although there were no significant differences, regarding CRP levels and NLR values, patients in the high group tended to have worse RFS than those in the low group (MST, CRP: 22.5 months in the high group vs. 23.3 months in the low group, p = 0.124; NLR: 22.4 months in the high group vs. 23.3 months in the low group, p = 0.198) (Online Resource 5).
Survival impact of diarrhea on the elderly
The effect of diarrhea on prognosis was then evaluated depending on the age. The median age of the enrolled patients was 69 years, and the patients were divided into four subgroups: younger (< 70 years) non-diarrhea group (Group N-Y, n = 55), elderly (≥ 70 years) non-diarrhea group (Group N-E, n = 51), younger diarrhea group (Group D-Y, n = 25), and elderly diarrhea group (Group D-E, n = 21) (Fig. 1). Patients in Group D-Y had significantly worse RFS than in Group N-Y (MST: 15.7 vs. 29.3 months, p = 0.022); nevertheless, there was no significant difference in OS between Groups D-Y and N-Y (MST: 24.1 vs. 33.6 months, p = 0.218) (Fig. 3c, d). However, patients in Group D-E had significantly worse OS and RFS than those in Group N-E (MST OS: 17.8 vs. 27.9 months, p < 0.001; RFS: 11.9 vs. 26.9 months, p < 0.001) (Fig. 3e, f). Finally, clinicopathological variables potentially affecting poor OS were investigated (Table 6). In the younger group, the multivariate analysis identified an advanced pathological stage (≥ III) (HR 4.732; p = 0.017; 95% CI 1.323–16.929) as an independent predictive factor for poor OS (Table 6). Diarrhea during EF was not associated with poor OS. However, in the elderly group, the multivariate analysis identified that an advanced pathological stage (≥ III) (HR 5.698; p = 0.016; 95% CI 1.391–23.335) and diarrhea during EF (HR 3.717; p = 0.040; 95% CI 1.059–13.042) were independent predictive factors for poor OS (Table 6).
Discussion
This study demonstrated that patients with diarrhea during EF after esophagectomy had significantly worse OS and RFS than those without diarrhea. Multivariate analysis revealed that diarrhea during EF was an independent prognostic factor for poor OS after esophagectomy. Furthermore, the nutritional status in Group D significantly decreased compared with that in Group N. To the best of our knowledge, this is the first report to reveal the survival impact of diarrhea during EF after esophagectomy.
Postoperative malnutrition has been reported to result in immune function deficiency and chemotherapy intolerance [5, 22]. Additionally, NLR increased only in Group D from the preoperative phase to 1 month after esophagectomy. Postoperative NLR values were similar between the postoperative infectious and non-infectious complication groups. Additionally, multivariate analysis identified that laparotomy and diarrhea during EF were relevant factors for high postoperative NLR values. These results suggested that postoperative neutrophil production was not associated with postoperative infectious complications but with diarrhea during EF. Furthermore, compared with that in the low NLR group, the Kaplan–Meier curve for RFS showed a decline in the high NLR group. Diarrhea during EF may induce inflammation, increasing neutrophil production and NLR elevations. Neutrophils induce chemokines and cytokine production, which enhances tumor growth, invasion, and angiogenesis [23]. These results suggest that diarrhea during EF can cause postoperative inflammation and malnutrition, resulting in decreased tumor immunity and a poor prognosis.
The stratified analysis revealed that RFS in the diarrhea group was significantly worse than that in the non-diarrhea group both in the younger and elderly groups. In addition, patients in the diarrhea group had significantly worse OS than those in the non-diarrhea group in the elderly group. Furthermore, the multivariate analysis revealed diarrhea during EF as an independent risk factor for poor OS in the elderly. Elderly patients usually have reduced satiety and poor digestive function, resulting in long-term reduced oral intake and malnutrition [11, 24]. Additionally, physiological function, especially organ reserve, declines with age, and comorbidities and frailty become increasingly common as people age. The decline in organ reserve becomes apparent only after stresses such as surgery or chemotherapy [25]. These findings suggest that diarrhea may have devastating effects on postoperative quality of life, physical strength, and prognosis, especially in the elderly.
Diarrhea during EF was associated with a higher dysphagia score. There is evidence that loss of oral intake can cause digestive and absorptive capacity deterioration due to intestinal villus atrophy [24, 26]. The esophageal cancer mass frequently obstructs the passage of solids before surgery, which causes atrophy of the intestinal villus and increases the risk of postoperative diarrhea.
Diarrhea can potentially disrupt the microbiota. By modulating the immune function of the host, intestinal bacteria can improve the immune system’s defense against cancer [27]. Intestinal microbiota disturbance can lead to the development of several pathologies, including malnourishment and chronic inflammatory disorders, such as inflammatory bowel disease, which have a significant impact on colorectal cancer pathogenesis [27, 28]. These findings suggest that diarrhea during EF may be a risk factor for intestinal microbiota distribution, malnutrition, and immunological disorders, contributing to a poor prognosis after esophagectomy.
This study found that there was no relationship between therapeutic antibiotic use and diarrhea. Antibiotics can negatively affect the gut microbiota, causing pathogenic bacteria, including Clostridioides difficile, to proliferate [29, 30]. However, few patients in this study had pathogenic bacteria that caused antibiotics-associated colitis, and resident bacteria in the intestinal tract were cultured in many patients. These findings suggest that diarrhea during EF was predominantly osmotic.
Diarrhea can be managed by slowing EF infusion or changing enteral nutritional supplements [31]. However, the frequency of diarrhea is often not accurately assessed and may be overlooked. Furthermore, an improvement in stool condition required several days from the start of diarrhea management in this study. Therefore, we formed a multidisciplinary support team and shared information on the nutritional status, dietary intake, selection of EF agents, and diarrhea at the conference [6, 18]. Regarding the administration of EF, it is important not only to adjust the rate of EF and agents according to abdominal symptoms but also to manage diarrhea during NAC. It is essential to communicate information about abdominal symptoms during EF with the multidisciplinary team and to administer prophylactic intestinal regimens.
This study has some limitations. First, this was a single-institution retrospective study. However, this study reviewed consecutive patients, which reduced selection bias. Second, there was some variability because the diagnosis of diarrhea was based on the subjective records of the nurse. In practice, there was a delay from detection to therapeutic intervention in some cases. To reduce bias among nurses, diarrhea was defined as the occurrence of continuous liquid stool for 3 days in this study. Finally, some patients were under treatment for recurrence at the conclusion of this study. Therefore, the effect of diarrhea on chemotherapy tolerance and the response rate for recurrence remains unclear. Continuous follow-ups and a multi-institutional prospective study should validate the current findings in the future.
In conclusion, diarrhea during EF can induce postoperative malnutrition, which leads to a poor prognosis after esophagectomy, especially in the elderly. Therefore, precise observation of the patient’s condition after esophagectomy may be essential in preventing EF-related diarrhea and a multidisciplinary support team may play an important role.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Yagi M, Hashimoto T, Nezuka H et al (1999) Complications associated with enteral nutrition using catheter jejunostomy after esophagectomy. Surg Today 29:214–218
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 16:1–24
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 16:25–43
Okadome K, Baba Y, Yagi T et al (2020) Prognostic nutritional index, Tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg 271:693–700
Haneda R, Hiramatsu Y, Kawata S et al (2022) Survival impact of perioperative changes in prognostic nutritional index levels after esophagectomy. Esophagus 19:250–259
Ryan AM, Rowley SP, Healy LA et al (2006) Post-oesophagectomy early enteral nutrition via a needle catheter jejunostomy: 8-year experience at a specialist unit. Clin Nutr 25:386–393
Tanaka Y, Yoshida K, Suetsugu T et al (2018) Recent advancements in esophageal cancer treatment in Japan. Ann Gastroenterol Surg 2:253–265
Weimann A, Braga M, Harsanyi L et al (2006) ESPEN Guidelines on enteral Nutrition: surgery including organ transplantation. Clin Nutr 25:224–244
Cataldi-Betcher EL, Seltzer MH, Slocum BA et al (1983) Complications occurring during enteral nutrition support: a prospective study. JPEN J Parenter Enteral Nutr 7:546–552
Gungabissoon U, Hacquoil K, Bains C et al (2015) Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr 39:441–448
Thibault R, Pichard C (2010) Nutrition and clinical outcome in intensive care patients. Curr Opin Clin Nutr Metab Care 13:177–183
Brierley JD (2017) TNM classification of malignant tumors, 8th edn. Blackwell, London
Kikuchi H, Hiramatsu Y, Matsumoto T et al (2020) The hybrid position is superior to the prone position for thoracoscopic esophagectomy with upper mediastinal lymphadenectomy. Ann Laparosc Endosc Surg 5:13–13
Booka E, Kikuchi H, Haneda R et al (2021) Short-term outcomes of robot-assisted minimally invasive esophagectomy compared with thoracoscopic or transthoracic esophagectomy. Anticancer Res 41:4455–4462
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Onodera T, Goseki N, Kosaki G (1984) Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85:1001–1005
Kawata S, Hiramatsu Y, Shirai Y et al (2020) Multidisciplinary team management for prevention of pneumonia and long-term weight loss after esophagectomy: a single-center retrospective study. Esophagus 17:270–278
Whelan K, Judd PA, Taylor MA (2004) Assessment of fecal output in patients receiving enteral tube feeding: validation of a novel chart. Eur J Clin Nutr 58:1030–1037
Yoon SR, Lee JH, Lee JH et al (2015) Low-FODMAP formula improves diarrhea and nutritional status in hospitalized patients receiving enteral nutrition: a randomized, multicenter, double-blind clinical trial. Nutr J 14:116
Laurent A, Marechal R, Farinella E et al (2022) Esophageal cancer: outcome and potential benefit of esophagectomy in elderly patients. Thorac Cancer 13:2699–2710
Reynolds JV, Kanwar S, Welsh FK et al (1997) Harry M. Vars Research Award. Does the route of feeding modify gut barrier function and clinical outcome in patients after major upper gastrointestinal surgery? JPEN J Parenter Enteral Nutr 21:196–201
Gregory AD, Houghton AM (2011) Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 71:2411–2416
Schiller LR (2009) Diarrhea and malabsorption in the elderly. Gastroenterol Clin North Am 38:481–502
Rostoft S, O’Donovan A, Soubeyran P et al (2021) Geriatric assessment and management in cancer. J Clin Oncol 39:2058–2067
Alpers DH (2002) Enteral feeding and gut atrophy. Curr Opin Clin Nutr Metab Care 5:679–683
Kich DM, Vincenzi A, Majolo F et al (2016) Probiotic: effectiveness nutrition in cancer treatment and prevention. Nutr Hosp 33:1430–1437
Cho I, Blaser MJ (2012) The human microbiome: at the interface of health and disease. Nat Rev Genet 13:260–270
Mullish BH, Williams HR (2018) Clostridium difficile infection and antibiotic-associated diarrhoea. Clin Med (Lond) 18:237–241
Jafarnejad S, Shab-Bidar S, Speakman JR et al (2016) Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18–64 years) but not the elderly (>65 years): A meta-analysis. Nutr Clin Pract 31:502–513
Whelan K, Schneider SM (2011) Mechanisms, prevention, and management of diarrhea in enteral nutrition. Curr Opin Gastroenterol 27:152–159
Acknowledgements
The authors gratefully acknowledge the work of the Hamamatsu Perioperative Care Team members for the collection of clinical data and supporting analysis.
Funding
No external funding was received for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ryoma Haneda, Yoshihiro Hiramatsu, and Sanshiro Kawata. The first draft of the manuscript was written by Ryoma Haneda and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures were conducted in accordance with institutional and national standards on human experimentation, as confirmed by the ethics committee of Hamamatsu University School of Medicine (approval number: 21-296), and the Declaration of Helsinki of 1964 and its later versions.
Informed consent
Informed consent was obtained from all the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Haneda, R., Hiramatsu, Y., Kawata, S. et al. Clinical impact of diarrhea during enteral feeding after esophagectomy. Int J Clin Oncol 29, 36–46 (2024). https://doi.org/10.1007/s10147-023-02428-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02428-5