Abstract
Background
Six-month adjuvant chemotherapy with S-1 is standard care for resected pancreatic cancer in Japan; however, the optimal duration has not been established. We aimed to evaluate the impact of duration of adjuvant chemotherapy with S-1.
Methods
We performed a multicenter, randomized, open-label, phase II study. Patients with histologically proven invasive pancreatic ductal carcinoma, pathological stage I–III, and no local residual or microscopic residual tumor were eligible. Patients were randomized 1:1 to receive 6- or 12-month adjuvant chemotherapy with S-1. The primary endpoint was 2-year overall survival (OS). Secondary endpoints were disease-free survival (DFS) and feasibility.
Results
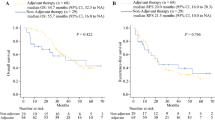
A total of 170 patients were randomized (85 per group); the full analysis set was 82 in both groups. Completion rates were 64.7% (6-month group) and 44.0% (12-month group). Two-year OS was 71.5% (6-month group) and 65.4% (12-month group) (hazard ratio (HR): 1.143; 80% confidence interval CI 0.841–1.553; P = 0.5758). Two-year DFS was 46.4% (6-month group) and 44.9% (12-month group) (HR: 1.069; 95% CI 0.727–1.572; P = 0.6448). In patients who completed the regimen, 2-year DFS was 56.5% (6-month group) and 75.0% (12-month group) (HR: 0.586; 95% CI 0.310–1.105; P = 0.0944). Frequent (≥ 5%) grade ≥ 3 adverse events comprised anorexia (10.5% in the 6-month group) and diarrhea (5.3% vs. 5.1%; 6- vs. 12-month group, respectively).
Conclusions
In patients with resected pancreatic cancer, 12-month adjuvant chemotherapy with S-1 was not superior to 6-month therapy regarding OS and DFS.



Similar content being viewed by others
References
Kobayashi K, Einama T, Takihata Y et al (2022) Therapeutic efficacy of dose-reduced adjuvant chemotherapy with S-1 in patients with pancreatic cancer: a retrospective study. BMC Cancer 22:1028
Itoh S, Tsujita E, Fukuzawa K et al (2021) Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: a multi-institutional retrospective study. Pancreatology 21:1356–1363
Sultana A, Smith CT, Cunningham D et al (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. J Clin Oncol 25:2607–2615
Sperti C, Pasquali C, Piccoli A et al (1997) Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg 21:195–200
Klinkenbijl JH, Jeekel J, Sahmoud T et al (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 230:776–782
Neoptolemos JP, Stocken DD, Friess H et al (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210
Oettle H, Post S, Neuhaus P et al (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277
Conroy T, Hammel P, Hebbar M et al (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395–2406
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388:248–257
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Yoshikawa T, Terashima M, Mizusawa J et al (2019) Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomized trial. Lancet Gastroenterol Hepatol 4:208–216
Japanese Pancreas Society (2009) General rules for the study of pancreatic cancer, 6th edn. Kanehara, Tokyo
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, Hoboken
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Common Terminology Criteria for Adverse Events v4.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40
Tomimaru Y, Eguchi H, Inoue Y et al (2023) Impact of S-1 adjuvant chemotherapy longer than 6 months on survival in patients with resected pancreatic cancer: a nationwide survey by the Japan Pancreas Society based on real-world data. Cancer 129:728–739
Valle JW, Palmer D, Jackson R et al (2014) Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 32:504–512
Yabusaki N, Fujii T, Yamada S et al (2016) The significance of relative dose intensity in adjuvant chemotherapy of pancreatic ductal adenocarcinoma including the analysis of clinicopathological factors influencing relative dose intensity. Medicine (Baltimore) 95:e4282
Tsukuda M, Kida A, Fujii M et al (2005) Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br J Cancer 93:884–889
Arai W, Hosoya Y, Hyodo M et al (2004) Alternate-day oral therapy with TS-1 for advanced gastric cancer. Int J Clin Oncol 9:143–148
Rino Y, Takanashi Y, Yukawa N et al (2006) A phase I study of bi-weekly combination therapy with S-1 and docetaxel for advanced or recurrent gastric cancer. Anticancer Res 26:1455–1462
Okumura N, Soh J, Suzuki H et al (2021) Randomized phase II study of daily and alternate-day administration of S-1 for adjuvant chemotherapy in completely-resected stage I non-small cell lung cancer: results of the Setouchi Lung Cancer Group Study 1301. BMC Cancer 21:506
Moriwaki T, Sakai Y, Ishida H et al (2019) Phase II study of S-1 on alternate days plus bevacizumab in patients aged ≥ 75 years with metastatic colorectal cancer (J-SAVER). Int J Clin Oncol 24:1214–1222
Ojima T, Nakamura M, Nakamori M et al (2019) Triplet chemotherapy with docetaxel, cisplatin and S-1 for unresectable advanced squamous cell carcinoma of the esophagus: phase I/II trial results. Oncotarget 10:847–855
Motoi F, Kosuge T, Ueno H et al (2019) Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 49:190–194
Acknowledgements
We thank Eisuke Adachi (Kyushu Central Hospital) and Yasuharu Ikeda (Fukuoka City Hospital) for the data and safety monitoring. We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HK: conceptualisation, data curation, methodology, formal analysis, investigation, resources, visualisation, writing – original draft, writing—review & editing. SI: conceptualisation, data curation, methodology, formal analysis, investigation, resources, visualization, project administration, writing—original draft, writing—review & editing. MS: conceptualisation, data curation, methodology, formal analysis, validation, writing—original draft, writing—review & editing. HH, HT, KF, MN, KA, Y-iY, KS, HU, YM, TU, TU, TM, HB: investigation, resources, writing—review & editing. TY: conceptualisation, methodology, project administration, supervision, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest regarding this article.
Ethical approval
The study protocol was approved by Kyushu University Hospital Institutional Review Board (Approval number: 25066). This trial was completed in accordance with the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies of the Ministry of Health, Labour, and Welfare of Japan.
Informed consent
Informed consent was obtained from all patients for inclusion in the study.
Registration number
This study was registered at University hospital Medical Information Network (UMIN) Clinical Trials Registry as UMIN000012634 (https://center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000014772).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kayashima, H., Itoh, S., Shimokawa, M. et al. Effect of duration of adjuvant chemotherapy with S-1 (6 versus 12 months) for resected pancreatic cancer: the multicenter clinical randomized phase II postoperative adjuvant chemotherapy S-1 (PACS-1) trial. Int J Clin Oncol 28, 1520–1529 (2023). https://doi.org/10.1007/s10147-023-02399-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02399-7