Background
TS-1 (1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyrimidine-1 M potassium oxonate) has a high single-agent response rate, of more than 40%, for gastric cancer; however, the recommended regimen of 4 weeks of administration interrupted by 2 weeks of drug withdrawal frequently causes adverse effects. The alternate-day dosage of pyrimidine fluoride anticancer drugs could reduce their adverse effects without compromising their effects. We attempted an alternate-day therapy with TS-1 aiming at the avoidance of adverse effects and significantly longer duration of administration.
Methods
We observed patients for clinical effects and adverse effects under alternate-day dosage of TS-1, and determined blood 5-fluorouracil (FU) levels. The judgment of clinical effects was based on the New Guidelines to Evaluate the Response to Treatment in Solid Tumors (RECIST), whereas the evaluation of adverse effects was based on the National Cancer Institute NCI-common toxicity criteria (CTC).
Results
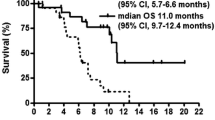
In 72 (78%) of 92 patients, the TS-1 regimen was converted to the alternate-day dosage because of adverse effects. Twenty patients were treated with the alternate-day dosage regimen from the start because of the fear of adverse effects. The alternate-day dosage was clinically effective, as 28 of 34 patients after relatively curative resection remained alive and free from recurrence. The median survival time of 58 patients after noncurative resection or with unresectable or recurrent cancer was 332 days. Fifty-three percent of these 58 patients achieved partial response and stable disease of more than 12 weeks’ duration. We followed time-dependent changes in blood 5-FU levels in 36 of the patients on alternate-day therapy, in whom TS-1 had been administered daily before being administered every other day. The trough level was significantly lower when TS-1 was administered on alternate days, and blood 5-FU reached a peak at sufficiently effective levels at 2 h even after administration on the alternate-day basis.
Conclusion
This study demonstrated that, compared with daily administration, alternate-day administration of TS-1 reduces adverse effects, and simultaneously ensures effective blood levels and provides sufficient clinical effects.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Arai, W., Hosoya, Y., Hyodo, M. et al. Alternate-day oral therapy with TS-1 for advanced gastric cancer. Int J Clin Oncol 9, 143–148 (2004). https://doi.org/10.1007/s10147-004-0381-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10147-004-0381-9