Abstract
Background
Although several randomized trials (RCTs) showed survival benefits of immune checkpoint inhibitor (ICI) plus first-line chemotherapy for advanced gastric or gastroesophageal cancer (AGC), these trials could enroll patients who fulfilled the strict eligibility criteria or waited for certain screening period for central assessment of PD-L1 status.
Methods
We retrospectively compared characteristics and clinical outcomes of the patients with AGC who received first-line chemotherapy in control arm of RCTs with ICIs (control group) or clinical practice (practice group) at our institution from February 2016 to April 2019.
Results
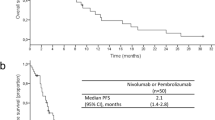
The control group had a better baseline Eastern Cooperative Oncology Group performance status (PS0, 81.2% vs. 51.4%, p < 0.001) and a longer interval from first visit to first-line chemotherapy initiation (19 days vs. 9 days, p < 0.001) than the practice group. Median overall survival (OS) was 20.3 months in control group and 15.7 months in practice group, with a trend of longer OS in control group than that in practice group (hazard ratio, 0.71; p = 0.062). More patients in control group were treated with subsequent chemotherapy including ICIs.
Conclusion
Patients with AGC in RCTs of ICIs had a better PS or a higher chance to receive subsequent chemotherapy, resulting in a better prognosis than those treated in clinical practice. This information should be considered when interpreting RCT results and applying new treatments into clinical practice.


Similar content being viewed by others
Data Availability
The evaluation data set analyzed in the current study is not available publicly. However, the data are available from the corresponding author on request.
References
Han JJ, Kim JW, Suh KJ et al (2019) Clinical characteristics and outcomes of patients enrolled in clinical trials compared with those of patients outside clinical trials in advanced gastric cancer. Asia Pac J Clin Oncol 15:158–165. https://doi.org/10.1111/ajco.13145
Unger JM, Barlow WE, Martin DP, et al (2014) Comparison of survival outcomes among cancer patients treated in and out of clinical trials. Jnci J National Cancer Inst 106:dju002. https://doi.org/10.1093/jnci/dju002
Go RS, Frisby KA, Lee JA et al (2006) Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer 106:426–433. https://doi.org/10.1002/cncr.21597
Elting LS, Cooksley C, Bekele BN et al (2006) Generalizability of cancer clinical trial results. Cancer 106:2452–2458. https://doi.org/10.1002/cncr.21907
Ohno S, Mukai H, Narui K et al (2019) Participants in a randomized controlled trial had longer overall survival than non-participants: a prospective cohort study. Breast Cancer Res Treat 176:631–635. https://doi.org/10.1007/s10549-019-05276-y
Braunholtz DA, Edwards SJL, Lilford RJ (2001) Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.” J Clin Epidemiol 54:217–224. https://doi.org/10.1016/s0895-4356(00)00305-x
Peppercorn JM, Weeks JC, Cook EF et al (2004) Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 363:263–270. https://doi.org/10.1016/s0140-6736(03)15383-4
Janjigian YY, Shitara K, Moehler M et al (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398:27–40. https://doi.org/10.1016/s0140-6736(21)00797-2
Shitara K, Cutsem EV, Bang Y-J et al (2020) efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer. Jama Oncol 6:1571–1580. https://doi.org/10.1001/jamaoncol.2020.3370
Kubota Y, Kawazoe A, Sasaki A et al (2020) The impact of molecular subtype on efficacy of chemotherapy and checkpoint inhibition in advanced gastric cancer. Clin Cancer Res 26:3784–3790. https://doi.org/10.1158/1078-0432.ccr-20-0075
Fuchs CS, Muro K, Tomasek J et al (2017) prognostic factor analysis of overall survival in gastric cancer from two phase iii studies of second-line ramucirumab (REGARD and RAINBOW) using pooled patient data. J Gastric Cancer 17:132–144. https://doi.org/10.5230/jgc.2017.17.e16
Takahari D, Boku N, Mizusawa J et al (2014) Determination of prognostic factors in japanese patients with advanced gastric cancer using the data from a randomized controlled trial, Japan clinical oncology group 9912. Oncologist 19:358–366. https://doi.org/10.1634/theoncologist.2013-0306
Kang Y-K, Chen L-T, Ryu M-H et al (2022) Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23:234–247. https://doi.org/10.1016/s1470-2045(21)00692-6
Kang Y-K, Boku N, Satoh T et al (2017) Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:2461–2471. https://doi.org/10.1016/s0140-6736(17)31827-5
Catenacci DVT, Kang Y-K, Saeed A et al (2021) FIGHT: A randomized, double-blind, placebo-controlled, phase II study of bemarituzumab (bema) combined with modified FOLFOX6 in 1L FGFR2b+ advanced gastric/gastroesophageal junction adenocarcinoma (GC). J Clin Oncol. https://doi.org/10.1200/jco.2021.39.15_suppl.4010
Nakamura Y, Taniguchi H, Ikeda M et al (2020) Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 26:1859–1864. https://doi.org/10.1038/s41591-020-1063-5
Kather JN, Pearson AT, Halama N et al (2019) Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med 25:1054–1056. https://doi.org/10.1038/s41591-019-0462-y
Yamashita R, Long J, Longacre T, et al Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. The Lancet Oncology 22: 132–141.
Acknowledgements
The authors thank all patients and families for participation in the studies, as well as colleagues. The authors also would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
Yu Aoki, Akihito Kawazoe, and Kohei Shitara contributed to the study conception, design, data analysis and interpretation. Material preparation and data collection were performed by Yu Aoki and Yohei Kubota. The first draft of the manuscript was written by Yu Aoki and Kohei Shitara. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yu Aoki declares no conflict of interest. Akihito Kawazoe reports honoraria from Daiichi Sankyo, Lilly, Ono, Taiho, Bristol Myers Squibb, and Merck Seronobiopharma: research funding from Ono, MSD, Taiho, Bayer, Sumitomo Dainippon and AstraZeneca. Yohei Kubota reports receiving research funding from Astellas. Keigo Chida reports honoraria from Bayer. Saori Mishima reports honoraria from Merck Seronobiopharma: research funding from Roche Diagnostics. Daisuke Kotani reports honoraria from Takeda, Chugai, Lilly, MSD, Ono, Taiho, Bristol Myers Squibb, and Merckbiopharma: research funding from Ono, MSD, Novartis, Servier, Janssen, IQVIA, Syneoshealth, and Cimicshiftzero. Yoshiaki Nakamura reports honoraria from Guardant Health Inc., Merck Pharmaceutical, and Chugai Pharmaceutical: research funding from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo, Guardant Health Inc., Genomedia Inc., Roche Diagnostics, Seagen outside the submitted work. Yasutoshi Kuboki reports receiving personal fees for advisory roles from Takeda, Boehringer Ingelheim, and Taiho: receiving honoraria (lecture fee) from ONO, Bayer, and Sanofi; and receiving research funding from Taiho, Takeda, ONO, Abbie, AstraZeneca, Boehringer Ingelheim, Incyte, Amgen, Chugai, GSK, Genmab, Astelas, and Daiichi-Sankyo, outside the submitted work. Hideaki Bando reports honoraria from Taiho Pharmaceutical, Eli Lilly Japan, and Ono Pharmaceutical: research funding from Ono Pharmaceutical and Chugai Pharmaceutical outside the submitted work. Takashi Kojima reports grants and personal fees from Ono Pharmaceutical and MSD, grants from Astellas Amgen BioPharma, Taiho Pharmaceutical and Shionogi, personal fees from Oncolys BioPharma, Astellas Pharma, BMS and Merk. Toshihiko Doi reports receiving personal fee for advisory roles from Boehringer Ingelheim, Janssen Pharmaceutical, Rakuten Medical, Amgen, MSD, Otsuka Pharmaceutical, Kyowa Kirin, Daiichi Sankyo, Sumitomo Dainippon, Abbvie, Takeda Pharmaceutical; receiving honoraria (lecture fee) from Ono Pharmaceutical, BMS and receiving research funding from TAIHO PHARMACEUTICAL, Novartis, MSD, Janssen Pharmaceutical, Boehringer Ingelheim, Eisai, Pfizer, Sumitomo Dainippon, Chugai Pharmaceutical, Daiichi Sankyo, BMS, Abbvie, Lilly, IQVIA, Merck Serono, outside the submitted work. Takayuki Yoshino reports honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly Japan, Merck Biopharma, Bayer Yakuhin, Ono Pharmaceutical and MSD; and receiving research funding from Ono Pharmaceutical, Sanofi, Daiichi Sankyo, PAREXEL International, Pfizer Japan, Taiho Pharmaceutical, MSD, Amgen, Genomedia Inc., Sysmex, Chugai Pharmaceutical and Nippon Boehringer Ingelheim, outside the submitted work. Takeshi Kuwata report honoraria from AstraZeneca, Celltrion, Astellas Pharma, MSD, Bristol-Myers Squibb Japan and Ono Pharmaceutical: Research funding from Roche Diagnostics Japan, Daiichi Sankyo and Ono Pharmaceutical. Kohei Shitara reports receiving personal fees for advisory roles from Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, Merck Pharmaceutical, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, Boehringer Ingelheim, and Janssen; receiving honoraria (lecture fee) from Takeda and Bristol-Myers Squibb; and receiving research funding from Astellas, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai, MSD, Medi Science, Eisai and Amgen, outside the submitted work.
Consent to participate
Informed consent requirement was waived due to the study's observational retrospective design, with an opt-out opportunity provided at the institution's website.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the institutional review board of the National Cancer Center Japan (No.2017-120).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Aoki, Y., Kawazoe, A., Kubota, Y. et al. Characteristics and clinical outcomes of patients with advanced gastric or gastroesophageal cancer treated in and out of randomized clinical trials of first-line immune checkpoint inhibitors. Int J Clin Oncol 27, 1413–1420 (2022). https://doi.org/10.1007/s10147-022-02200-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02200-1