Abstract
Background
UGT1A1 polymorphisms should be considered when using irinotecan-containing regimens, especially in patients with a double-variant-type (DV), including homozygous for UGT1A1*28 and UGT1A1*6 and heterozygous for both UGT1A1*28 and UGT1A1*6. We investigated the safety and efficacy of modified FOLFIRINOX (mFOLFIRINOX) (irinotecan 80 mg/m2) in patients having DV.
Methods
Patients with advanced pancreatic cancer who had received FOLFIRINOX between January 2015 and December 2019 were included in this study. Non-DV patients received the standard mFOLFIRINOX (irinotecan 150 mg/m2) as first-line (non-DV1) or second-line therapy (non-DV2); however, DV patients received mFOLFIRINOX (irinotecan 80 mg/m2) as the second-line therapy (DV2). We retrospectively evaluated the safety and efficacy of the lowered irinotecan dose in the DV2 group relative to the non-DV1 (safety) or non-DV2 (safety and efficacy) groups.
Results
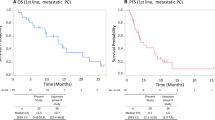
A total of 235 patients were eligible for this study with 118 patients in the non-DV1, 106 in the non-DV2, and 11 in the DV2 groups. Major grade 3–4 adverse events were neutropenia (33.9, 31.1, and 18.2%) and febrile neutropenia (6.8, 3.8, and 9.1%) in the non-DV1, non-DV2, and DV2 groups, respectively. The median progression-free survival was 3.4 months in the non-DV2 group, and 4.4 months in the DV2 group. The overall survival from the date of starting second-line chemotherapy was 8.8 months in the non-DV2 group and 11.5 months in the DV2 group.
Conclusions
Based on our findings, the safety and efficacy of mFOLFIRINOX (irinotecan 80 mg/m2) in DV patients were comparable with the standard mFOLFIRINOX (irinotecan 150 mg/m2) in non-DV patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to data from the World Health Organization (WHO), in 2020, pancreatic ductal adenocarcinoma (PDAC) was the twelfth most common cancer (495,773 incident cases) and the seventh leading cause of cancer-related deaths (466,003 deaths) [1]. In Japan, PDAC is the fourth leading cause of cancer-related death, and the mortality rate is increasing annually [2]. PDAC is expected to be the second leading cause of cancer-related deaths in developed countries in the next few years [3, 4].
PDAC has a poor prognosis, and the US “Cancer Statistics, 2019” suggests the lowest five-year relative survival rate for PDAC at 9% for all stages and only 3% for metastatic disease (the most common form) [4]. In metastatic or locally advanced PDAC, patients cannot undergo surgery; therefore, systemic therapy can be used to prolong life expectancy and improve (or preserve) quality of life.
With the results of the PRODIGE 4/ACCORD 11 trial, FOLFIRINOX (every two weeks: oxaliplatin 85 mg/m2 d1, irinotecan 180 mg/m2, leucovorin 400 mg/m2, 5-fluorouracil (5-FU) 400 mg/m2, and 5-fluorouracil 2400 mg/m2 over 46 h) has become a first-line therapeutic option for metastatic PDAC [5]. FOLFIRINOX offers a survival benefit over gemcitabine monotherapy [median overall survival (OS) 11.1 months vs. 6.8 months, P < 0.001] but with high rates of grade 3–4 adverse events, such as neutropenia, febrile neutropenia, thrombocytopenia, diarrhea, and sensory neuropathy.
In clinical practice, many oncologists reduce the dose of one or more of the FOLFIRINOX components (5-FU, oxaliplatin, or irinotecan) to minimize toxicity. Ozaka et al. reported a phase II trial of modified FOLFIRINOX (mFOLFIRINOX) to evaluate its tolerability and efficacy [6], which involved a dose reduction of irinotecan to 150 mg/m2 and no intravenous bolus injection of 5-FU. Herein, the incidences of grade 3–4 neutropenia and febrile neutropenia were less than those reported previously in the original FOLFIRINOX study in Japan [7]. The efficacy of the 150 mg/m2 irinotecan dose was also as high as that of the original FOLFIRINOX [median OS 11.2 months and objective response rate (ORR) 37.7%] [6]. Therefore, mFOLFIRINOX is now commonly used for the treatment of advanced PDAC.
Irinotecan is a prodrug; its active metabolite (SN-38) shows the antitumor activity as well as toxicity. The enzyme UDP-glucuronosyltransferase 1 family polypeptide A1 (UGT1A1), encoded by UGT1A1, glucuronidates SN-38 to SN-38-G (an inactive metabolite). UGT1A1 has germline polymorphisms such as UGT1A1*28 and UGT1A1*6. The wild-type allele (UGT1A1*1) has six TA repeats in the promoter region but the variant allele (UGT1A1*28) has seven TA repeats. Another variant allele (UGT1A1*6) consists of a single nucleotide replacement in exon 1 of the UGT1A1 gene. UGT1A1*6 is important because it is one of the major polymorphisms among the Asian population but is rarely found in Caucasians [8]. Patients with genetic polymorphisms in UGT1A1 have a dose-dependent increase in the risk of grade 3–4 hematologic toxicity and diarrhea [8,9,10]. Patients homozygous for UGT1A1*28 (UGT1A1*28/*28), homozygous for UGT1A1*6 (UGT1A1*6/*6), and heterozygous for both UGT1A1*28 and UGT1A1*6 (UGT1A1*6/*28) are defined as having double-variant-type (DV) UGT1A1 polymorphisms. In Japan, the frequency of DV is approximately 10%, comparable to that of UGT1A1*28 homozygotes in Caucasian populations [11, 12].
A dose reduction for DV patients with PDAC should be considered but the recommended initial dose of mFOLFIRINOX remains undetermined. Minami et al. have suggested that the dose of irinotecan for DV patients should be reduced to half of that recommended for non-DV patients. This is considering a 2.4-fold steep relationship between the dose of irinotecan and the AUC of SN-38 for DV patients compared with non-DV patients [12]. Sharma et al. have reported that the reduction of irinotecan dose to 90 mg/m2 in mFOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 90 mg/m2, leucovorin 400 mg/m2, and 5-FU 2400 mg/m2 over 46 h) was intolerable, and thus not feasible for patients with the UGT1A1*28/*28 genotype [13].
Considering these results, we hypothesized that further dose reduction of irinotecan was needed for DV patients. In this study, we, therefore, examined the effects of a reduced irinotecan dose of 80 mg/m2 in clinical practice for retrospectively evaluating the safety and efficacy of mFOLFIRINOX.
Patients and methods
Patients
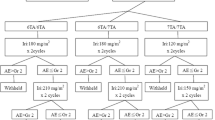
The subjects were patients with unresectable or recurrent pancreatic cancer who received FOLFIRINOX therapy at the National Cancer Center Hospital (NCCH) and were screened for UGT1A1 genetic polymorphisms between January 2015 and December 2019. All subjects underwent a UGT1A1 polymorphism test during diagnosis. We divided the patients into three groups according to UGT1A1 polymorphism and treatment line. Patients with the DV possessed the UGT1A1*6/*6, UGT1A1*28/*28, and UGT1A1*6/*28 genotypes and received mFOLFIRINOX as second-line therapy (DV2). The other patients without DV received mFOLFIRINOX as first-line (non-DV1) or second-line therapy (non-DV2). Patients with DV treated with mFOLFIRINOX as first-line therapy were excluded because there was only one patient in this group (Fig. 1).
Methods
Treatment
DV patients received reduced dose of mFOLFIRINOX (every 2 weeks: oxaliplatin 85 mg/m2, irinotecan 80 mg/m2, leucovorin 200 mg/m2, and 5-FU 2400 mg/m2 over 46 h). Although this was not a prospective study, considering the previous reports of Minami et al. and Sharma et al., irinotecan dose was reduced to 80 mg/m2 for all-DV patients who chose mFOLFIRINOX as second-line therapy during the study period at our institute. We explained our strategy of treatment to the DV patients using a reduced dose of mFOLFIRINOX; a few patients wanted mFOLFIRINOX treatment without the reduced dose of irinotecan. Non-DV patients received the standard mFOLFIRINOX (every two weeks: oxaliplatin 85 mg/m2, irinotecan 150 mg/m2, leucovorin 200 mg/m2, and 5-FU 2400 mg/m2 over 46 h). Non-DV patients who had received mFOLFIRINOX with an initial dose of irinotecan (180 or 120 mg/m2) were excluded from this study (Fig. 1). Prophylactic filgrastim or pegfilgrastim in cycle 1 was not permitted.
Assessment
This was a retrospective, single-center study conducted in NCCH and approved by the institutional review boards of the NCCH. Clinical data of the patients were obtained from the electronic medical records. The collected clinical data included age; sex; Eastern Cooperative Oncology Group performance status (ECOG-PS); extent of PDAC disease (recurrence, locally advanced, metastatic); an initial dose of irinotecan; first and last administration date of mFOLFIRINOX; type and severity of adverse events; and date of death [14]. We evaluated the safety and efficacy of irinotecan at a dose of 80 mg/m2 in the DV2 group in comparison with those of the non-DV1 (safety) or non-DV2 (safety and efficacy) groups. Regarding safety, grade ≥ 3 adverse events during the entire cycle of mFOLFIRINOX in the DV2 group were recorded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events v5.0 (CTCAE 5.0) and compared with those in non-DV1 and non-DV2 groups [15]. As for efficacy, we focused on second-line treatment cases, because we had only one case of first-line treatment in all of the DV patients and excluded it from the study. We, therefore, assessed the responses of progression-free survival (PFS) and overall survival only in second-line treatment cases. Tumor response was analyzed using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16]. The proportion of patients showing response was calculated as the total proportion of patients with a best overall response, complete or partial; the proportion of patients with disease control was calculated as the total proportion of patients with a best overall response, complete or partial, or with stable disease. Survival duration and occurrence frequencies of adverse events were analyzed up to February 2022. At this cutoff time, one patient in the DV2 group and five in the non-DV2 group were alive. The median length of follow-up was 10.5 months (range, 0.3–60.5) for all subjects.
Statistical analysis
PFS was defined as the period from the start of second-line mFOLFIRINOX treatment to tumor progression or death. OS was defined as the period from the start of second-line mFOLFIRINOX treatment to death. Categorical variables were compared using Fisher’s exact test. The median values of the variables were compared using the Mann–Whitney U test. Time-to-event data were analyzed using standard methods, including Kaplan–Meier product-limit estimates, and compared between independent groups using the log-rank test. Statistical significance was set at P < 0.05. Statistical analysis was performed using EZR software version 1.38 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [17].
Results
Patient characteristics
A total of 235 patients were eligible for this study consisting of 224 patients with non-DV PDAC and 11 patients with DV PDAC. In the non-DV group, 118 patients (49.7%) received mFOLFIRINOX as first-line (non-DV1), and 106 patients (41.3%) as second-line (non-DV2) (Fig. 1) therapies. The patient characteristics are shown in Table 1. The median age at the initiation of mFOLFIRINOX was 62 years in the non-DV1, 62 years in the non-DV2, and 64 years in the DV2 groups. The proportion of female patients was higher in the DV group than in the non-DV group. The ECOG-PS score was 0 or 1 in almost all patients. Regarding the extent of disease, 66 patients (55.9%) in the non-DV1, 60 patients (56.6%) in non-DV2, and 5 patients (45.5%) in the DV2 groups had metastatic disease. In contrast, 42 patients (35.6%) in the non-DV1, 28 patients (26.4%) in the non-DV2, and 5 patients (45.5%) in the DV2 groups had locally advanced disease, while 10 patients (8.5%) in the non-DV1, 18 patients (17.0%) in the non-DV2, and 1 patient (9.1%) in the DV2 groups had a postoperative recurrence. In the DV2 group, the genotypes of UGT1A1 were *6/*6 in two patients (18.2%), *28/*28 in one patient (9.1%), and *6/*28 in eight patients (72.7%). Whereas the genotypes of UGT1A1 were *1/*1 in 62 non-DV1 patients (52.5%) and 54 of non-DV2 patients (50.9%); *1/*6 in 34 non-DV1 patients (28.8%) and 30 of non-DV2 patients (28.3%); and *1/*28 in 22 non-DV1 patients (18.6%) and 22 of non-DV2 patients (20.8%). The initial oxaliplatin dose was reduced only for two patients in the non-DV2 group, whereas in the non-DV1 and DV2 groups, dosages were not reduced. The initial 5-FU dose was not reduced for any patient. All patients in the DV2 group and 100 patients (94.3%) in the non-DV2 group received the first-line treatment with gemcitabine plus nab-paclitaxel. Other regimens for first-line treatment are shown in Table 1. The median number of mFOLFIRINOX treatment cycles was 13 in the non-DV1, 9 in the non-DV2, and 12 in the DV2 group.
Adverse events
The grade 3–4 hematological and non-hematological adverse events reported in > 5% of patients are summarized in Table 2. In the non-DV1 and non-DV2 groups, major adverse events were neutropenia (33.9, 31.1%), febrile neutropenia (6.8, 3.8%), diarrhea (11.9, 2.8%), peripheral sensory neuropathy (9.3, 2.8%), and anorexia (7.6, 0.9%), respectively. In the DV2 group, neutropenia and febrile neutropenia were observed in 18.2% and 9.1% of the patients, respectively, and grade 3–4 adverse events, including diarrhea, peripheral sensory neuropathy, and anorexia, were not observed. Prophylactic filgrastim or pegfilgrastim was not used for all subjects. During mFOLFIRINOX treatment, filgrastim was administered to eight patients (6.8%) in the non-DV1, one (0.9%) in the non-DV2, and none in the DV2 group, as a treatment for febrile neutropenia. Interstitial lung disease (ILD) was found in one patient (0.8%) in the non-DV1, two (1.9%) in the non-DV2, and none in the DV2 groups. Although these ILD cases were of grade 1 or 2, mFOLFIRINOX treatment was discontinued in all three patients.
Efficacy
The treatment efficacy was evaluated using the best overall response based on the RECIST criteria and compared only in patients who received mFOLFIRINOX as second-line therapy. In the non-DV2 group, 1 patient (0.9%) had complete response, 13 patients (12.3%) had partial responses (PR), 39 patients (36.8%) had stable disease (SD), 52 patients (49.1%) had progressive disease (PD), and 1 patient (0.9%) was not evaluable for response. In the DV2 group, one patient (9.1%) had PR, six patients (54.5%) had SD, and four patients (36.4%) had PD. No significant difference in response rate (13.2% vs. 9.1%) or disease control rate (50.0% vs. 63.6%) was obtained between the non-DV2 and DV2 groups, respectively. The median PFS from the date of starting second-line chemotherapy was 3.4 months (95% CI 2.5–4.7) in the non-DV2 group and 4.4 months (95% CI 1.7–11.3) in the DV2 group. The median OS from the date of starting second-line chemotherapy was 8.8 months (95% CI 7.3–10.6) in the non-DV2 group and 11.5 months (95% CI 4.4–19.5) in the DV2 group. There was no significant difference in PFS (P = 0.417) or OS (P = 0.579) between the non-DV2 and DV2 groups (Fig. 2). In the non-DV1 group, 40 patients (33.9%) had PR, 54 patients (45.8%) had SD, 21 patients (17.8%) had PD, and 3 patients (0.9%) were not evaluated for response, while the disease control rate was 79.7%. The median PFS and OS from the date of starting first-line chemotherapy were 7.8 months (95% CI 5.9–10.1) and 16.2 months (95% CI 14.8–19.5).
Discussion
FOLFIRINOX is a preferred treatment regimen for PDAC recommended in the NCCN guidelines [18]. However, it induces high-grade toxicity, such as myelosuppression, GI toxicity, and neuropathy. Therefore, mFOLFIRINOX, less toxic than the original FOLFIRINOX, is also a preferred regimen recommended in the NCCN guidelines and is commonly used worldwide. Although the UGT1A1 polymorphism should be taken into consideration when choosing an irinotecan dose in treatments such as FOLFIRINOX, there is currently a paucity of evidence for a preferable dose reduction.
The safety of FOLFIRINOX in PDAC patients with wild and heterozygous UGT1A1*6 and *28 polymorphisms was assessed in Japanese patients by Shirasu et al. [19]. There was no difference in the frequency of adverse events depending on the UGT1A1 status in patients with wild and heterozygous genotypes. Umemoto et al. suggested that an initial dose of irinotecan ≤ 120 mg/m2 is possibly the optimal dose for the first cycle of FOLFIRINOX for Japanese DV patients with advanced PDAC [20]. In this study, 11 patients were administered the 90–100 mg/m2 irinotecan dose. Within this subgroup, grade 4 neutropenia was observed in 27% of patients, and grade 3–4 adverse events, such as febrile neutropenia, diarrhea, and anorexia, were observed in 18% of patients. These results were negative for the safety of FOLFIRINOX with reduced irinotecan dose (90–100 mg/m2). In another study, Sharma et al. reported that dose-limiting toxicity occurred in 4/10 (40%) in DV (UGT1A1*28/*28) patients who received mFOLFIRINOX with a 90 mg/m2 irinotecan dose, and this dose reduction was not tolerable for DV patients with pancreatic and biliary tract cancers [13]. Minami et al. suggested that the dose of irinotecan for DV patients should be reduced to half of the dosage recommended for other patients, considering a 2.4-fold steep relationship between the dose of irinotecan and the AUC of SN-38 for DV patients compared with non-DV patients [12]. Based on these results, we hypothesized that further dose reduction of irinotecan is needed for DV patients with PDAC. Consequently, our study showed a milder toxicity profile than those previously reported for DV patients and a Japanese phase II study for non-DV patients (Table 3) [6, 13, 20]. These results from our study indicated that mFOLFIRINOX with a reduced dose of irinotecan at 80 mg/m2 is tolerable for DV2 patients. Moreover, non-DV1 patients had slightly more grade 3–4 adverse events than non-DV2 patients. This result may indicate a selection bias. Non-DV2 and DV2 patients in our study were not treated with milder chemotherapy such as nanoliposomal irinotecan in combination with 5-FU and folinic acid or S-1 monotherapy, but by mFOLFIRINOX as second-line therapy. These non-DV2 and DV2 patients might be in better general condition than non-DV1 patients. This bias could also occur in DV patients; therefore, if we use mFOLFIRINOX as first-line therapy for DV patients, more attention should be paid to toxicity. As for febrile neutropenia (FN), granulocyte colony-stimulating growth factor (G-CSF) decreases the risk of FN in patients receiving myelosuppressive chemotherapies. A recent systematic review showed that prophylactic use of G-CSF reduces the rates of FN, dose reduction, and treatment delay [21]. Our group previously conducted a phase II trial to evaluate the safety and efficacy of primary prophylactic pegfilgrastim in patients with metastatic pancreatic cancer who received FOLFIRINOX [22]. However, FN occurred in 18.0% and previously, we could not demonstrate the efficacy of pegfilgrastim addition to FOLFIRINOX; the DV patients were excluded. Thus, the efficacy of prophylactic pegfilgrastim in DV patients needed elucidation.
For efficacy, we assessed second-line treatment cases because we had only one case for first-line treatment in all-DV patients (excluded from the study). Along with FOLFIRINOX, gemcitabine + nab-paclitaxel (GnP) treatment is another preferred first-line treatment of PDAC. For patients treated with GnP or other gemcitabine-based regimens as first-line treatment, nanoliposomal irinotecan + 5-FU / leucovorin treatment as a second-line treatment showed preferable results in phase III (NAPOLI-1) study and became one of the recommended second-line treatment regimens. This NAPOLI-1 regimen achieved an ORR of 16%, a disease control rate of 52%, while median PFS was 3.1 months, and OS was 6.1 months [23]. FOLFIRINOX is another treatment option for second-line treatment after gemcitabine-related regimens, especially for patients with good PS. In Table 4, the results from major previous reports for second-line FOLFIRINOX are shown together with our study results [24,25,26,27,28,29]. In our study, there were no significant differences between the DV2 and non-DV2 groups, not only in treatment response and disease control but also in PFS and OS. Relative to previous findings, our DV2 group did not show a high response rate; however, the disease control rate was better. Although other study results could not be compared without considering patient characteristics, mFOLFIRINOX with a reduced dose of irinotecan at 80 mg/m2 likely showed as high efficacy as a previous study with FOLFIRINOX and NAPOLI-1 trials. As for first-line mFOLFIRINOX therapy, we showed strong results not only in safety but also in efficacy in non-DV1 patients.
To the best of our knowledge, this study is the first to report the safety and efficacy of mFOLFIRINOX with a fixed, reduced dose of irinotecan (80 mg/m2) for DV patients. In addition, our results showed the safety and efficacy of second-line mFOLFIRINOX in a large number of patients, including both DV and non-DV, and we have discussed how our results were comparable with previous results. However, this study had some limitations. It was a single-center retrospectively conducted study, and the sample size was small. The UTG1A1 genotype difference in DV patients could have some effects on safety and efficacy. In our study, the genotypes of UGT1A1-DV were almost all *6/*28 double heterozygous type, and only a few patients had *6/*6 (two patients) or *28/*28 homozygous type (one patient). This proportion in the UGT1A1 genotype differed from those in the Umemoto and Sharma studies [13, 20]. As for efficacy, we evaluated only second-line treatment cases. In our institute, most DV patients received GnP or other gemcitabine-related regimens for first-line treatment, because we have limited data about the safety and efficacy of mFOLFIRINOX as first-line treatment in DV patients.
In conclusion, the results of our study indicated that 80 mg/m2 irinotecan mFOLFIRINOX was relatively well tolerated, suggesting that it may serve as a second-line treatment option for DV patients with PDAC. Our results also demonstrated relatively better disease control efficacy as second-line therapy. Further studies are necessary to evaluate the safety and efficacy of first-line treatment for DV patients with PDAC.
References
The Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf Accessed on 6 Feb 2021.
Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Ministry of Health, Labour and Welfare, national cancer registry).
Rahib L, Smith BD, Aizenberg R et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 74:2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
Rawla P, Sunkara T, Gaduputi V (2019) Epidemiology of pancreatic cancer: global trends, etiology and risks factors. World J Oncol 10:10–27. https://doi.org/10.14740/wjon1166
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Ozaka M, Ishii H, Sato T et al (2018) A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 81:1017–1023. https://doi.org/10.1007/s00280-018-3577-9
Okusaka T, Ikeda M, Fukutomi A et al (2014) Phase II study of FOLFIRINOX for chemotherapy-Naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 105:1321–1326. https://doi.org/10.1111/cas.12501
Ando Y, Saka H, Ando M et al (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60:6921–6926
Hoskins JM, Goldberg RM, Qu P et al (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99:1290–1295. https://doi.org/10.1093/jnci/djm115
Innocenti F, Undevia SD, Iyer L et al (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388. https://doi.org/10.1200/JCO.2004.07.173
Akiyama Y, Fujita K, Nagashima F et al (2008) Genetic testing for UGT1A1*28 and *6 in Japanese patients who receive irinotecan chemotherapy. Ann Oncol 19:2089–2090. https://doi.org/10.1093/annonc/mdn645
Minami H, Sai K, Saeki M et al (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics 17:497–504. https://doi.org/10.1097/FPC.0b013e328014341f
Sharma MR, Joshi SS, Karrison TG et al (2019) A UGT1A1 genotype-guided dosing study of modified FOLFIRINOX in previously untreated patients with advanced gastrointestinal malignancies. Cancer 125:1629–1636. https://doi.org/10.1002/cncr.31938
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
National Comprehensive Cancer Network. Pancreatic adenocarcinoma (version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 22 Aug 2021
Shirasu H, Todaka A, Omae K et al (2019) Impact of UGT1A1 genetic polymorphism on toxicity in unresectable pancreatic cancer patients undergoing FOLFIRINOX. Cancer Sci 110:707–716. https://doi.org/10.1111/cas.13883
Umemoto K, Takahashi H, Morizane C et al (2021) FOLFIRINOX in advanced pancreatic cancer patients with the double-variant type of UGT1A1*28 and *6 polymorphism: a multicenter, retrospective study. Cancer Chemother Pharmacol 87:397–404. https://doi.org/10.1007/s00280-020-04206-w
Lyman GH, Dale DC, Culakova E et al (2013) The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol 24:2475–2484. https://doi.org/10.1093/annonc/mdt226
Sasaki M, Ueno H, Mitsunaga S et al (2021) A phase II study of FOLFIRINOX with primary prophylactic pegfilgrastim for chemotherapy-Naïve Japanese patients with metastatic pancreatic cancer. Int J Clin Oncol 26:2065–2072. https://doi.org/10.1007/s10147-021-02001-y
Wang-Gillam A, Li CP, Bodoky G et al (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial NAPOLI-1 study group. Lancet 6(387):545–557. https://doi.org/10.1016/S0140-6736(15)00986-1
Kobayashi N, Omae K, Horita Y et al (2020) FOLFIRINOX as second-line chemotherapy for advanced pancreatic cancer: a subset analysis of data from a nationwide multicenter observational study in Japan. Pancreatology 20:1519–1525. https://doi.org/10.1016/j.pan.2020.07.006
Foschini F, Napolitano F, Servetto A et al (2020) FOLFIRINOX after first-line gemcitabine-based chemotherapy in advanced pancreatic cancer: a retrospective comparison with FOLFOX and FOLFIRI schedules. Ther Adv Med Oncol 12:1758835920947970. https://doi.org/10.1177/1758835920947970
Sawada M, Kasuga A, Mie T et al (2020) Modified FOLFIRINOX as a second-line therapy following gemcitabine plus nab-paclitaxel therapy in metastatic pancreatic cancer. BMC Cancer 20:449. https://doi.org/10.1186/s12885-020-06945-8
Kim JH, Lee SC, Oh SY et al (2018) Attenuated FOLFIRINOX in the salvage treatment of gemcitabine-refractory advanced pancreatic cancer: a phase II study. Cancer Commun (Lond) 38:32. https://doi.org/10.1186/s40880-018-0304-1
Saito K, Nakai Y, Takahara N et al (2021) A retrospective comparative study of S-IROX and modified FOLFIRINOX for patients with advanced pancreatic cancer refractory to gemcitabine plus nab-paclitaxel. Investig New Drugs 39:605–613. https://doi.org/10.1007/s10637-020-01022-0
Chung MJ, Kang H, Kim HG et al (2018) Multicenter phase II trial of modified FOLFIRINOX in gemcitabine-refractory pancreatic cancer. World J Gastrointest Oncol 10:505–515. https://doi.org/10.4251/wjgo.v10.i12.505
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takuji Okusaka reports a consulting/advisory relationship with Taiho Pharmaceutical, Daiichi Sankyo, Sumitomo Dainippon Pharma, Bristol Myers Squibb, AstraZeneca, Eisai, and Nihon Servier; research funding from Eli Lilly Japan, MSD, AstraZeneca, Eisai, Chugai Pharmaceutical, Novartis Pharma, Sumitomo Dainippon Pharma, Bristol Myers Squibb, Taiho Pharmaceutical, and Baxter; honoraria from Meiji Seika Pharma, Teijin Pharma, Eli Lilly Japan, MSD, AstraZeneca, AbbVie, Eisai, Ono Pharmaceutical, Yakult Honsha, Daiichi Sankyo, Chugai Pharmaceutical, Nihon Servier, and Novartis Pharma; and scientific advisory board fees from AstraZeneca, Nippon Shinyaku, Nihon Servier, Novartis Pharma, Pfizer, and Mundipharma. Chigusa Morizane has received a speaker honorarium from Nihon Servier and Yakult Honsha. Akihiro Ohba has received a speaker honorarium from Ono Pharmaceutical, Taiho Pharmaceutical, and Yakult Honsha, and research funding from Ono Pharmaceutical, and Chugai Pharmaceutical. The other authors declare that they have no conflict of interest.
Ethics approval
This study was conducted in accordance with the principles laid down in the 1964 Declaration of Helsinki and its later amendments, and the protocol was approved by the Ethics Committee of the National Cancer Center (Approval No. 2018-149) and all the institutions participating in this study. Approval for review of the hospital records was obtained from the Institutional Review Board of the National Cancer Center and the need for informed consent from the patients was waived in view of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Satake, T., Morizane, C., Maruki, Y. et al. The influence of UGT1A1 polymorphisms on modified FOLFIRINOX dose in double-variant-type patients with advanced pancreatic cancer. Int J Clin Oncol 27, 1331–1339 (2022). https://doi.org/10.1007/s10147-022-02186-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02186-w