Abstract
Management of non-metastatic castration-resistant prostate cancer (nmCRPC) has undergone a paradigm shift with next-generation androgen receptor inhibitors. However, direct comparative data are not available to inform treatment decisions and/or guideline recommendations. Therefore, we performed network meta-analysis to indirectly compare the efficacy and safety of currently available treatments. Multiple databases were searched for articles published before June 2020. Studies that compared overall and/or metastasis-free and/or prostate-specific antigen (PSA) progression-free survival (OS/MFS/PSA-PFS) and/or adverse events (AEs) in nmCRPC patients were considered eligible. Three studies (n = 4117) met our eligibility criteria. Formal network meta-analyses were conducted. For MFS, apalutamide, darolutamide, and enzalutamide were significantly more effective than placebo, and apalutamide emerged as the best option (P score: 0.8809). Apalutamide [hazard ratio (HR): 0.85, 95% credible interval (CrI): 0.77–0.94] and enzalutamide (HR: 0.86, 95% CrI: 0.78–0.95) were both significantly more effective than darolutamide. For PSA-PFS, all three agents were statistically superior to placebo, and apalutamide emerged as the likely preferred option (P score: 1.000). Apalutamide (HR: 0.71, 95% CrI: 0.69–0.74) and enzalutamide (HR: 0.76, 95% CrI: 0.74–0.79) were both significantly more effective than darolutamide. For AEs (including all AEs, grade 3 or grade 4 AEs, grade 5 AEs, and discontinuation rates), darolutamide was the likely best option. Apalutamide and enzalutamide appear to be more efficacious agents for therapy of nmCRPC, while darolutamide appears to have the most favorable tolerability profile. These findings may facilitate individualized treatment strategies and inform future direct comparative trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most common solid cancer and the second most common cause of cancer-related death in men [1]. Systemic therapy based on androgen deprivation is the standard primary treatment strategy in patients with advanced prostate cancer. Despite adequate therapy, the disease eventually progresses to castration-resistant prostate cancer (CRPC) [2]. While docetaxel has long been the only agent with level 1 evidence for improved overall survival (OS) in metastatic CRPC (mCRPC), the advent of novel drugs/treatments, such as enzalutamide, abiraterone acetate, cabazitaxel, sipuleucel-T, and radium-223 has revolutionized therapeutic strategies for mCRPC [3,4,5,6,7,8,9,10].
CRPC without any metastases on conventional imaging is classified as non-metastatic CRPC (nmCRPC); preventing or delaying progression to metastatic disease is an area of unmet clinical need among patients with nmCRPC [11]. Recently, the phase III PROSPER, SPARTAN, and ARAMIS trials conducted in patients with high risk nmCRPC demonstrated enzalutamide, apalutamide, or darolutamide to be associated with a significantly longer median metastasis-free survival (MFS) compared to placebo [12,13,14]. Based on these results, guidelines have recommended them in patients with nmCRPC with a prostate-specific antigen doubling time (PSADT) of less than 10 months [15].
However, the available data directly comparing the effectiveness and safety of these agents are scarce to inform optimal treatment decisions and guideline recommendations. Moreover, long-term results of the PROSPER trial were recently reported [16]. Therefore, we conducted a systematic review of all clinical trials assessing treatment with next-generation androgen receptor inhibitors for nmCRPC using placebo as the control arm, and performed network meta-analyses to indirectly compare the efficacy and safety of these agents.
Methods
Search strategy
The systematic review and network meta-analysis of randomized controlled trials (RCTs) comparing systemic therapies for nmCRPC (with placebo as the control arm) were conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) extension statement for network meta-analysis [17]. The PubMed, Web of Science, and Scopus databases were searched to identify reports published until June 2020 on systemic therapy for nmCRPC. The following keywords were used in our search strategy: (prostate carcinoma OR prostate cancer OR prostatic carcinoma OR prostatic cancer) AND (non-metastatic OR no metastatic OR M0) AND (castration resistant OR castration refractory OR hormone refractory OR hormone resistant) AND (Randomized). The primary outcome of interest was MFS, and the secondary outcomes were PSA-PFS, OS, and adverse events (AEs). Initial screening was performed independently by two investigators based on the titles and abstracts of the article to identify ineligible reports. Reasons for exclusions were noted. Potentially relevant reports were subjected to a full-text review, and the relevance of the reports was confirmed after the data extraction process. Disagreements were resolved via consensus with the co-authors.
Inclusion and exclusion criteria
Studies were included if they investigated nmCRPC patients (Patients) who had undergone systemic therapy (Intervention) compared with those treated with placebo (Comparison) to assess the differential effects on MFS, PSA-PFS, OS, and AEs (Outcome) in a randomized studies only. We excluded observational studies, reviews, letters, editorials, meeting abstracts, replies from authors, case reports, and articles not published in English. References of all papers included were scanned for additional studies of interest. Studies were included only if they involved patients who received placebo as the control arm.
Data extraction
Two investigators independently extracted the following information from the included articles: first author’s name, publication year, period of patient recruitment, number of patients, treatment dosage, age, study design, oncologic outcomes, and AE outcomes. Subsequently, the hazard ratios (HR) and 95% confidence intervals (CI) associated with MFS, PSA-PFS and OS, and AE rate were retrieved. HRs were extracted from cox analyses. All discrepancies regarding data extraction were resolved by consensus with the co-authors.
Risk of bias assessment
The “risk-of-bias” (RoB) evaluation of each study was assessed according to The Cochrane Collaboration’s tool for assessing risk of bias [18]. This tool assesses selection bias (random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other sources of bias (Supplementary Figure. 1). The RoB of each study was assessed independently by two authors. Disagreements were resolved by consultation with the co-authors.
Statistical analyses
MFS was defined as the time from randomization to the first detection of distant metastasis on imaging or death. For each outcome, we conducted network meta-analysis using random and fixed effect models with a Bayesian approach for the direct and indirect treatment comparisons with placebo as the common comparator arm [19, 20]. In the assessment for MFS, PSA-PFS and OS, contrast-based analyses were applied with estimated differences in the log HR and the standard error calculated from the published HR and CI[21]. The relative treatment effects were presented as HR and 95% credible interval (CrI) [19]. With regard to MFS, subgroup analyses were conducted among: PSADT ≤ 6 M and PSADT > 6 M. For the assessment of the AEs, arm-based analyses were performed to estimate odds ratios (OR) of the AEs (and 95% CrI) from the available raw data presented in the selected manuscripts [19]. We also estimated the relative ranking of the different treatments for each outcome by using the P score, which can be considered a frequentist analog to the surface under the cumulative ranking curves [22, 23]. Network plots were utilized to illustrate the connectivity of the treatment networks in terms of MFS, PSA-PFS, OS, and AEs. Heterogeneity was assessed using I2 when more than one trial was available for a given comparison. All statistical analyses were performed using R 3.6.3 and Stata/MP 14.2 (Stata Corp., College Station, TX); statistical significance was set at P < 0.05.
Results
Study selection and characteristics
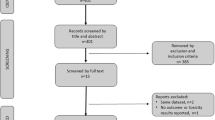
Our initial search identified 1205 publications, and after the elimination of duplicates, a total of 1057 publications were available. A total of 1036 articles were excluded after screening the titles and abstracts, and a full-text review was performed for 21 articles (Fig. 1). Based on the selection criteria, we identified 3 articles comprising 4117 patients for the systematic review and network meta-analysis [12,13,14, 16, 24]. Extracted data from the three studies are outlined in Table 1. All these studies were published between 2018 and 2020 and included a total of 1423 patients (median age: 73–74 years, median PSADT: 3.6–4.7 months) treated with placebo and 2694 patients (median age: 74 years, median PSADT: 3.8–4.4 months) treated with a next-generation androgen receptor inhibitors.
Network meta-analysis
The networks of eligible comparisons are graphically represented in network plots in terms of MFS, PSA-PFS, OS, and AEs (Supplementary Figure. 2).
MFS
A network meta-analysis of 3 different agents was conducted for the primary outcome of MFS. Compared with placebo, apalutamide, darolutamide, and enzalutamide resulted in a significantly improved MFS (HR: 0.58, 95% CrI: 0.54–0.61, HR: 0.68, 95% CrI: 0.63–0.74, and HR: 0.58, 95% CrI: 0.55–0.62, respectively) (Fig. 2a). Compared with darolutamide, apalutamide and enzalutamide resulted in a significantly improved MFS (HR: 0.85, 95% CrI: 0.77–0.94, and HR: 0.86, 95% CrI: 0.78–0.95, respectively). Based on analysis of the treatment ranking, apalutamide had the highest likelihood of providing the maximal MFS (P score: 0.8809), closely followed by enzalutamide (P score: 0.7852) (Table 2).
PSA PFS
A network meta-analysis of three different agents was conducted for the secondary outcome of PSA-PFS. Compared with placebo, apalutamide, darolutamide, and enzalutamide resulted in a significantly improved PSA-PFS (HR: 0.29, 95% CrI: 0.29–0.30, HR: 0.41, 95% CrI: 0.40–0.42, and HR: 0.32, 95% CrI: 0.31–0.32, respectively) (Fig. 2b). Compared with darolutamide, apalutamide and enzalutamide resulted in a significantly improved PSA-PFS (HR: 0.71, 95% CrI: 0.69–0.74, and HR: 0.76, 95% CrI: 0.74–0.79, respectively). Based on analysis of the treatment ranking, apalutamide had the highest likelihood of providing the maximal PSA-PFS (P score: 1.0000) followed by enzalutamide (P score: 0.6667) (Table 2).
OS
A network meta-analysis of three different agents was conducted for the secondary outcome of OS. Compared with placebo, apalutamide, darolutamide, and enzalutamide did not result in a significantly improved OS (HR: 0.87, 95% CrI: 0.76–1.00, HR: 0.86, 95% CrI: 0.67–1.10, and HR: 0.88, 95% CrI: 0.73–1.06, respectively) (Fig. 2c). Based on analysis of the treatment ranking, apalutamide had the highest likelihood of providing the maximal OS (P score: 0.6594), closely followed by darolutamide and enzalutamide (P score: 0.6589 and 0.6024, respectively) (Table 2).
AEs
A network meta-analysis of three different agents was conducted for the various outcomes of AEs (including any AE, grade 3 or grade 4 AE, grade 5 AE, and discontinuation rates).
Darolutamide caused similar number of Grade 5 AEs and discontinuation compared with placebo (OR: 1.20, 95% CrI: 0.68–2.13, and OR: 1.03, 95% CrI: 0.71–1.49, respectively). By contrast, apalutamide (OR: 5.01, 95% CrI: 0.64–39.25, and OR: 1.56, 95% CrI: 1.00–2.44, respectively) and enzalutamide (OR: 5.49, 95% CrI: 1.67–18.02 and OR: 1.61, 95% CrI: 1.04–2.50, respectively) were associated with a significantly higher likelihood of grade 5 and toxicity leading to discontinuation (Fig. 3a, b). All three drugs were associated with a significantly higher likelihood of toxicity regarding any AEs and grade 3 or grade 4 AEs (Fig. 3c, d). Based on analysis of the treatment ranking, it was highly likely that darolutamide had the lowest rate of all AE outcomes compared to both apalutamide and enzalutamide (Supplementary Table 1).
MFS (PSADT ≤ 6 M)
Compared with placebo, in patients with PSADT ≤ 6 M nmCRPC, apalutamide, darolutamide, and enzalutamide resulted in significantly improved MFS (HR: 0.58, 95% CrI: 0.55–0.62, HR: 0.68, 95% CrI: 0.62–0.75, and HR: 0.58, 95% CrI: 0.54–0.61, respectively) (Supplementary Figure. 3A). Compared with darolutamide, apalutamide and enzalutamide resulted in significantly improved MFS. Based on analysis of the treatment ranking, enzalutamide had the highest likelihood of providing the maximal MFS (P score: 0.8768), closely followed by apalutamide (P score: 0.7875) (Supplementary Table 2).
MFS (PSADT > 6 M)
Compared with placebo, in patients with PSADT > 6 M nmCRPC, apalutamide, darolutamide, and enzalutamide resulted in significantly improved MFS (HR: 0.59, 95% CrI: 0.52–0.68, HR: 0.66, 95% CrI: 0.57–0.76, and HR: 0.63, 95% CrI: 0.53–0.75, respectively) (Supplementary Figure. 3B). Based on analysis of the treatment ranking, apalutamide had the highest likelihood of providing the maximal MFS (P score: 0.8574) (Supplementary Table 2).
Discussion
We conducted systematic review on systemic therapy agents that have been evaluated in placebo-controlled RCTs for patients with nmCRPC; we also performed network meta-analysis to indirectly compare the safety and efficacy of these therapies. This approach generated several important findings. First, only new androgen receptor inhibitors were adequately tested in this clinical disease space. Second, apalutamide emerged as the most likely best treatment option with regard to MFS and PSA PFS. Moreover, apalutamide and enzalutamide were significantly more effective than darolutamide with regards to MFS and PSA PFS. Conversely, third, darolutamide was the best-tolerated of all three agents evaluated causing a similar number of AEs compared to placebo with regard to grade 5 AE rate and discontinuation rate.
These developments are of particular interest, as previous network meta-analyses did not include recently reported data [25] [26]. Therefore, we included recently published data, such as updated results from the PROSPER and SPARTAN trials [16, 24]. In addition, this network meta-analysis included detailed AE outcomes. This is of greater relevance to clinical practice than the recent network meta-analyses [25, 26]. On these points, current paper may more readily facilitate individualized treatment selection.
Results from the ARAMIS, PROSPER, and SPARTAN trials showed positive effects of apalutamide, darolutamide, or enzalutamide, respectively, on the primary endpoint MFS [12,13,14]. Despite finding similar efficacy in MFS compared to placebo, investigators from the PROSPER trial included only patients without lymph node enlargement (N0) while the SPARTAN and ARAMIS trials included patients with lymph nodes up to 2 cm in diameter in the short axis (N1) below aortic bifurcation. Subgroup analyses in both trials indicated a potential benefit for apalutamide and darolutamide for patients with N1 status compared to N0 (HR 0.15 vs. 0.33 and HR 0.28 vs. 0.46, respectively) suggests that the efficacy of enzalutamide may have been unfairly estimated in comparison with the other agents. However, this possibility must be validated and investigated in more detail to obtain reliable data on the relevance of lymph node positivity and the therapeutic efficacy of these agents. Moreover, the inclusion criteria differed somewhat, with a minimum serum PSA of 2 ng/ml in ARAMIS and PROSPER, and no minimum in SPARTAN. Despite these differences, the present network meta-analysis indicated that apalutamide may be the most effective therapeutic option based on its benefits with regard to MFS and PSA PFS. Apalutamide is molecularly and mechanistically similar to enzalutamide, as it antagonizes the ligandbinding domain of androgen receptor (AR) with potent affinity, thereby preventing AR nuclear translocation; it also does not have agonistic effects in the presence of AR overexpression [27]. Apalutamide led to ≥ 50% tumor regression in eight of ten castrate immunodeficient mice harboring LNCaP/AR xenograft tumors, whereas bicalutamide led to ≥ 50% tumor regression in only one of the ten evaluated mice [27]. Notably, apalutamide also demonstrated greater in vivo activity in CRPC xenograft models [28], compared to enzalutamide, 2–4-times lower doses of apalutamide were needed to achieve stable, therapeutic plasma concentrations in a mouse model of human CRPC xenografts with approximately the same drug concentrations in the tumor; this suggests a higher therapeutic index for apalutamide with a greater scope for dose escalation [27].
Another important finding from this meta-analysis was that darolutamide was the best tolerated of all three evaluated agents. Darolutamide is a non-steroidal AR-antagonist with a molecular structure that is distinct from those of enzalutamide and apalutamide [29]. In general, darolutamide has advantage of fewer and less severe toxic effects than apalutamide and enzalutamide because of its low penetration of the blood–brain barrier and low-binding affinity for g-aminobutyric acid type A receptors based on preclinical studies [30, 31]. Indeed, darolutamide was associated with fewer central nervous system effects than either enzalutamide or apalutamide with respect to seizures (enzalutamide 11% and apalutamide 15.6% vs. darolutamide 4.2%), cognitive/mental impairment disorders (enzalutamide 5% and apalutamide 5.1% vs. darolutamide 0.4%) or dizziness (enzalutamide 10% and apalutamide 9.3% vs. darolutamide 4.5%). In addition, it is important to point out that patients with known central nerves system malignancies were included in the ARAMIS study, while they were excluded in PROSPER and SPARTAN. Moreover, both enzalutamide and apalutamide are strong CYP3A4 inducers and thus have potential for CYP-mediated drug-drug interactions such as decreased plasma exposure to warfarin via CYP inhibition [32]. By contrast, darolutamide is not a CYP-inhibitor and is, therefore, less likely to cause drug-drug interactions than enzalutamide or apalutamide [33].
Despite the comprehensive nature of this systematic review, there are some limitations that need to be considered when interpreting the results. First, although indirect treatment comparison analyses have been used and validated for comparing outcomes from RCTs, this approach falls short of a head-to-head treatment comparison. Thus, direct well designed comparative trials are required to validate the findings of this study. Second, this network meta-analysis was based on the reporting quality of the trials we reviewed and may have been affected by several types of biases, thus limiting the validity of the overall findings. Third, the patient characteristics may have differed significantly between the studies, thereby limiting the comparability of the trials evaluated. Indeed, as mentioned above, caution should be exercised in assessing the data on enzalutamide as the inclusion criteria for lymph node status differed from those applied in the other studies. Moreover, because of inherent limitations of published data, performing a meta-analysis of adjusted effect estimates proved to be impossible. Finally, differences in subsequent therapies received across the treatment arms in the trials evaluated may have potentially influenced the OS results. In addition, the OS data from some trials were still immature; thus, the study outcomes could change in their final analyses. In addition to these limitations, accurate distinction between non-metastatic and metastatic disease based solely on conventional imaging modalities could be problematic owing to their limited sensitivity. Interestingly, over 90% of men with nmCRPC determined according to conventional imaging were ultimately found to have metastases using prostate-specific membrane antigen positron emission tomography in retrospective series [34]. Hence, patients in the three trials analyzed in the present study should be considered to have low-volume metastatic disease rather than nmCRPC, and the primary endpoint of progression to metastases on conventional imaging seems less relevant as prostate-specific membrane antigen positron emission tomography imaging is increasingly used in clinical settings.
Despite these caveats, the current network meta-analysis suggests that apalutamide may be the most efficacious option for the treatment of nmCRPC while darolutamide seems the safest. Although this meta-analysis does not replace the need for head-to-head clinical trials of contemporary systemic therapies, this finding could help improve clinical decision-making until such direct comparative data become available.
Conclusion
In this systematic review and network meta-analysis of first-line systemic therapies for patients with nmCRPC, based on an indirect comparison of data from placebo controlled phase 3 clinical trials, apalutamide was identified as having a higher likelihood of providing the maximum benefits in terms of MFS and PSA PFS. Darolutamide appeared to have the most favorable tolerability. These findings may provide guidance to patients and clinicians with regards to treatment decisions in conjunction with other aspects that drive personalized medicine strategies for nmCRPC.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA. Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Kluth LA, Shariat SF, Kratzik C et al (2014) The hypothalamic-pituitary-gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J Urol 32(3):669–676. https://doi.org/10.1007/s00345-013-1157-5
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med 351(15):1502–1512. https://doi.org/10.1056/NEJMoa040720
Beer TM, Armstrong AJ, Rathkopf DE et al (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J med 371(5):424–433. https://doi.org/10.1056/NEJMoa1405095
Scher HI, Fizazi K, Saad F et al (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med 367(13):1187–1197. https://doi.org/10.1056/NEJMoa1207506
Ryan CJ, Smith MR, de Bono JS et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl J Med 368(2):138–148. https://doi.org/10.1056/NEJMoa1209096
de Bono JS, Logothetis CJ, Molina A et al (2011) Abiraterone and increased survival in metastatic prostate cancer. New Engl J Med 364(21):1995–2005. https://doi.org/10.1056/NEJMoa1014618
Oudard S (2011) TROPIC: Phase III trial of cabazitaxel for the treatment of metastatic castration-resistant prostate cancer. Future Oncol 7(4):497–506. https://doi.org/10.2217/fon.11.23
Kantoff PW, Higano CS, Shore ND et al (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med 363(5):411–422. https://doi.org/10.1056/NEJMoa1001294
Hoskin P, Sartor O, O’Sullivan JM et al (2014) Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 15(12):1397–1406. https://doi.org/10.1016/s1470-2045(14)70474-7
Mateo J, Fizazi K, Gillessen S et al (2019) Managing nonmetastatic castration-resistant prostate cancer. Eur Urol 75(2):285–293. https://doi.org/10.1016/j.eururo.2018.07.035
Hussain M, Fizazi K, Saad F et al (2018) Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. New Engl J Med 378(26):2465–2474. https://doi.org/10.1056/NEJMoa1800536
Smith MR, Saad F, Chowdhury S et al (2018) Apalutamide treatment and metastasis-free survival in prostate cancer. New Engl J Med 378(15):1408–1418. https://doi.org/10.1056/NEJMoa1715546
Fizazi K, Shore N, Tammela TL et al (2019) Darolutamide in nonmetastatic, castration-resistant prostate cancer. New Engl J Med 380(13):1235–1246. https://doi.org/10.1056/NEJMoa1815671
Mottet N, van den Bergh RCN, Briers E et al (2019) EAU Guidelines: Prostate Cancer 2019. https://uroweb.org/guideline/prostate-cancer/
Sternberg CN, Fizazi K, Saad F et al (2020) Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. New Engl J Med 382(23):2197–2206. https://doi.org/10.1056/NEJMoa2003892
Hutton B, Salanti G, Caldwell DM et al (2015) The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 162(11):777–784. https://doi.org/10.7326/m14-2385
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
van Valkenhoef G, Lu G, de Brock B et al (2012) Automating network meta-analysis. Res Synth Methods 3(4):285–299. https://doi.org/10.1002/jrsm.1054
Dias S, Sutton AJ, Ades AE et al (2013) Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 33(5):607–617. https://doi.org/10.1177/0272989x12458724
Woods BS, Hawkins N, Scott DA (2010) Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 10:54. https://doi.org/10.1186/1471-2288-10-54
Rucker G, Schwarzer G (2015) Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 15:58. https://doi.org/10.1186/s12874-015-0060-8
Salanti G, Ades AE, Ioannidis JP (2011) Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 64(2):163–171. https://doi.org/10.1016/j.jclinepi.2010.03.016
Small EJ, Saad F, Chowdhury S et al (2019) Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol Off J Eur Soc Med Oncol 30(11):1813–1820. https://doi.org/10.1093/annonc/mdz397
Hird AE, Magee DE, Bhindi B et al (2020) A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2020.02.005
Kumar J, Jazayeri SB, Gautam S et al (2020) Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: a systematic review and network meta-analysis. Urol Oncol. https://doi.org/10.1016/j.urolonc.2020.03.022
Clegg NJ, Wongvipat J, Joseph JD et al (2012) ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res 72(6):1494–1503. https://doi.org/10.1158/0008-5472.can-11-3948
Tran C, Ouk S, Clegg NJ et al (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324(5928):787–790. https://doi.org/10.1126/science.1168175
Borgmann H, Lallous N, Ozistanbullu D et al (2018) Moving towards precision urologic oncology: targeting enzalutamide-resistant prostate cancer and mutated forms of the androgen receptor using the novel inhibitor darolutamide (ODM-201). Eur Urol 73(1):4–8. https://doi.org/10.1016/j.eururo.2017.08.012
Moilanen AM, Riikonen R, Oksala R et al (2015) Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep 5:12007. https://doi.org/10.1038/srep12007
Zurth C, Sandmann S, Trummel D et al (2018) Blood-brain barrier penetration of [14C] darolutamide compared with [14C] enzalutamide in rats using whole body autoradiography. J Clin Oncol 36:345–345. https://doi.org/10.1200/JCO.2018.36.6_suppl.345(6_suppl)
Hebenstreit D, Pichler R, Heidegger I (2019) Drug-drug interactions in prostate cancer treatment. Clin Genitourin Cancer. https://doi.org/10.1016/j.clgc.2019.05.016
Fizazi K, Massard C, Bono P et al (2017) Safety and antitumour activity of ODM-201 (BAY-1841788) in castration-resistant, CYP17 inhibitor-naive prostate cancer: results from extended follow-up of the ARADES trial. Eur Urol Focus 3(6):606–614. https://doi.org/10.1016/j.euf.2017.01.010
Fendler WP, Weber M, Iravani A et al (2019) Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin Cancer Res 25(24):7448–7454. https://doi.org/10.1158/1078-0432.ccr-19-1050
Funding
Open access funding provided by Medical University of Vienna. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Project development: KM, PIK, TK, SE, SFS. Data collection: KM, HM. Data analysis: KM, HM. Manuscript writing/editing: KM, HM, BP, RSM, FQ, EL, VMS, MA, PIK, TK, SE, SFS.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mori, K., Mostafaei, H., Pradere, B. et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol 25, 1892–1900 (2020). https://doi.org/10.1007/s10147-020-01777-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01777-9