Abstract
Spatial patterns in aggregations form as a result of the interplay between costs and benefits experienced by individuals. Such self-organisation of aggregations can be explained using a zonal model in which a short-range zone of repulsion and longer-range zone of attraction surrounding individuals leads to emergent pattern properties. The signal of these processes can be detected using spatial pattern analyses. Furthermore, in sessile organisms, post-settlement mortality reveals the relative costs and benefits of positions within the aggregation. Acorn barnacles are known to require contact with conspecifics for reproduction and are therefore believed to aggregate for this purpose; isolated individuals may also be more susceptible to abiotic stress and predation. At short distances, however, competition for space and resources is likely to occur. In this study spatial patterns of barnacles (Semibalanus balanoides L.) were analysed using pair-correlation functions. Individuals were dispersed at distances below 0.30 cm, but peak relative density occurred at a distance of 0.36 cm from conspecifics. This is much closer than required for reproductive access, implying a strong aggregative drive, up to the point of physical contact with neighbours. Nevertheless, analysis of dead barnacles illustrated that such proximity carries a cost as barnacles with many neighbours were more likely to have died. The inferences obtained from these patterns are that barnacles aggregate as closely as they can, and that local neighbourhood competition is a powerful determinant of mortality. These processes give rise to the observed pattern properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggregations of organisms are ubiquitous in nature, spanning from prokaryotes to multicellular eukaryotes (Parrish et al. 2002). They differ greatly in form between species. Some have thousands of individuals, others only several; some are transient (de Bono et al. 2002) while others are obligate (Nathan and Muller-Landau 2000); some are dynamic (Parrish and Edelstein-Keshet 1999) while others are static (Nathan and Muller-Landau 2000). Aggregations are maintained because the average fitness payoff to an individual in an aggregation is greater than the average payoff to a solitary individual, resulting in positive selection for grouping behaviour (Parrish and Edelstein-Keshet 1999).
Non-random spatial patterns within aggregations form due to the same behaviours as those forming the aggregations themselves: they are the result of individual cost-benefit trade-offs, with each individual acting to increase its own fitness. Given the complexity of biological systems, it is difficult to predict the spatial patterns which will occur within aggregations, and even once observed, the cost-benefit interplay which causes the resultant patterns is not easily discerned. Spatial pattern analysis therefore attempts to identify whether non-random patterns are occurring, and to test hypotheses regarding the proximate reasons as to why the pattern has emerged (Illian et al. 2008).
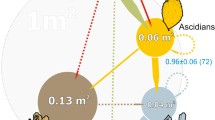
Spatial patterns of aggregating organisms can be clustered, random, over-dispersed or regular (Couzin et al. 2002). These self-organised patterns can appear as though individuals are aware of and responding to the entire group. However, individual units interacting in space need only obey a set of simple, local neighbourhood rules in order for complex patterns across multiple scales to form (Giardina 2008). Couzin and Krause (2003) proposed that interaction rules could be described zonally, with zones radiating outwards from the individual (Fig. 1). Immediately surrounding each individual is the zone of repulsion, within which individuals move apart due to the costs of close proximity. Beyond this is a zone of attraction within which individuals move towards one another due to benefits of aggregation.
Illustration of spatial processes determining settlement behaviour and survival of barnacles with distance to nearest conspecific from a given individual (black dot). Interior circle (dark shading) corresponds to the Zone of Repulsion (sensu Couzin and Krause 2003) and is determined by short-scale negative effects of proximity. Exterior circle (pale shading) corresponds to the Zone of Attraction and is determined by longer-scale positive effects of aggregation. The point at which zones switch from repulsion to attraction reflects the balance between the scale and strength of interactions
In sessile organisms, individuals cannot respond to changing costs and benefits by adjusting their position relative to neighbours. Settlement decisions made by the dispersal stage of the organism’s lifecycle determine not only the immediate fitness payoff to the organism but also the subsequent costs and benefits it will be exposed to. In some cases (e.g., seeds) the settlement process is passive, and non-random spatial patterns arise as the result of dispersal ability and abiotic factors such as wind direction. In other cases (e.g., barnacle larvae) settlement is both active and passive. Larval distribution and settlement location is dependent on dispersal ability and abiotic factors at large-scales, for example oceanic currents (Southward 1987), but at smaller scales is influenced by larval behaviour (Satumanatpan and Keough 2001). Patterns are then modulated by post-settlement mortality.
Here we focus on the barnacle Semibalanus balanoides, a boreo-arctic species which inhabits the lower intertidal zone of rocky shores (Jenkins et al. 2000). Several factors determine the spatial distribution of adults: larval distribution, larval settling behaviour, and post-settlement mortality. Semibalanus balanoides starts life as a pelagic nauplius larva, which hatches in spring and spends some weeks travelling in the water column (Bertness et al. 1991). After passing through six instars it transforms into a cyprid larva, which must find a suitable place to settle before metamorphosing into a sessile, filter-feeding adult (Southward 1987). Larval abundance is determined by a myriad of biotic and abiotic factors, including resources, oceanic currents and temperature, and is therefore decoupled from local adult population density (Bertness et al. 1991). As a result, densities of adults within sites may change drastically between years (Hills and Thomason 2003).
Cyprids actively search for suitable settlement locations on rocky shores by crawling over the substratum. Where they settle is highly dependent on the location of both adult and larval conspecifics which they detect through chemical cues (Pawlik 1992). Site choice by settlers is unlikely to be the dominant process determining final patterns, however, as 99.7 % of settlers die before reaching reproductive age (Pineda et al. 2006). Adults are almost always clustered, which is thought to be primarily because they are obligate cross-fertilisers and need to be within reproductive range of other individuals, a range determined by the maximum penis length of 2.5 cm (Barnes and Crisp 1956; Kent et al. 2003). Other potential benefits of proximity include structural facilitation, protection from predation, decreased hiding time, thermal buffering, and protection from wave exposure (Fig. 1; Connell 1961; Wethey 1984; Bertness 1989; Mauck and Harkless 2001; Kent et al. 2003).
Proximity to other barnacles carries costs which include competition for space causing decreased body size, which is a primary predictor of fertilisation success (Wu 1980); basal-membrane displacement, where individuals are undercut by neighbours, leading to desiccation via membrane exposure; or crushing by conspecifics (Connell 1961; Wethey 1983; Hui and Moyse 1987). Increased competition for mates also reduces individual fitness at high population densities (Charnov 1980). These costs are thought to result in the territoriality, or zone of repulsion, observed in S. balanoides populations (Connell 1961; Crisp 1961).
In this study we perform a test of the zonal model proposed by Couzin and Krause (2003) (Fig. 1) through analyses of the spatial patterns of live and dead S. balanoides barnacles within aggregations. Ecological processes reveal themselves through their spatial signatures (Watt 1947), and the recent development of spatial point pattern analyses has opened up the potential to yield insights into the interactions among individuals (Illian et al. 2008), though within ecological research to date these tools have been largely applied to plants (e.g., Law et al. 2009). Barnacles, as sessile organisms, provide an ideal test case for the existence of similar processes among invertebrates. Through this work we find evidence of both zones of repulsion and attraction, and provide direct estimates of the scales at which these occur. This shows that barnacles most frequently locate as close as they can to conspecifics without overlapping, but that this close contact carries the risk of subsequent mortality.
Materials and methods
Study site
Trearddur Bay is a sheltered beach in west Anglesey, North Wales (UK National Grid Reference: SH 25559 79014; Fig. 2). In the Anglesey area, peak tidal range is around 5 m, and monthly mean sea temperatures vary from 7 °C (February) to 16 °C. Two sites on rocky shores were selected based on ease of access and relative topological homogeneity. Site 1 was 32 × 44 m, and Site 2 was 24 × 26 m. Both sites were 60 m inland and were fully submerged at high tide. Random 20 × 20 cm quadrats were used to obtain samples of barnacle spatial patterns within each site. A top-down photograph of each was taken at a perpendicular angle using a Canon EOS 1100D. For this study images were chosen for which the surface was flat and homogenous, and no barnacle species other than S. balanoides were present. This yielded a total of six quadrats in Site 1 and three from Site 2.
Data preparation and analysis
Locations of individual barnacles were obtained using the PointPicker tool (Thévanez 2008) within the image processing software ImageJ (Rasband 1997). Dead barnacles were identified by lack of opercular plates. Diameters were measured across the widest point of each barnacle, other than for quadrat 1b, where high density of barnacles prevented accurate measurements. Spatial point patterns were then analysed using the spatstat package (Baddeley and Turner 2005) in R 3.2.0 (R Development Core Team 2015). Analysis employed the pair correlation function g(r) (Illian et al. 2008; Law et al. 2009), a robust descriptor obtained from the first derivative of Ripley’s K function (Ripley 1977), which gives the expected number of points within a distance r summed across all points in the pattern and divided by its average density λ. This is estimated within a given window as:
where r is the distance from each point i, \(I_{ij}(r)\) is 1 for each j within r of i and otherwise 0, and n is the total number of points. This provides a cumulative function which can be converted to the pair correlation function \(g(r) = \frac{{K}'(r)}{2\pi r}\). In ecological terms it describes the neighbourhood density at increasing distance r. If densities are independent at a given distance, \(g(r) \approx 1\). When \(g(r) > 1\), pairs of individuals are more abundant than the spatial average, while \(g(r) < 1\) indicates that they are less abundant. Following Baddeley and Turner (2005), g(r) was estimated up to a quarter of the minimum plot dimension, i.e., 5 cm. Detecting departure of patterns from complete spatial randomness (CSR) requires the construction of envelopes based on null model simulations. In this study envelopes illustrate the fifth-ranked highest and lowest of 999 Monte Carlo simulations of a homogeneous Poisson distribution of the same intensity as the empirical pattern. This is equivalent to a two-tailed test with \(\alpha \approx 0.01\). Though not a formal test of significance, the approach is strongly supported by most authors (Illian et al. 2008; Law et al. 2009).
The cross-pair correlation function \(g_{ij}(r)\) is a natural extension of g(r) above in which i and j refer to different types of points; in this case to dead and live barnacles respectively. This therefore allows for testing of whether the pattern of live barnacles around dead barnacles differs from that expected by chance. Careful choice of null models is essential to ensure adequate tests of spatial patterning hypotheses (Wiegand and Moloney 2004). In this study the positions of dead barnacles were kept fixed while the positions of live barnacles were shifted using a random toroidal transformation. This allowed us to test whether live and dead barnacles are independently distributed whilst maintaining their inherent spatial patterns. In all cases envelopes were calculated as above based on 999 iterations of the null model. Four sites were excluded from analysis of post-settlement mortality patterns due to inadequate sample sizes (<20) of dead individuals.
In order to assess the degree of competition experienced by barnacles within each quadrat we used the method of Deevey (1947) to calculate the average number of contact points individual barnacles have with neighbours. This is calculated as \(C = 2\pi r^2(\frac{n}{A})^2\) where C is the crowding coefficient, r is the average radius of individuals, n is the number of individuals and A is the total area of study. We then compare this to Deevey’s own results.
Results
Numbers of barnacles within 20 × 20 cm quadrats varied by an order of magnitude from 331 to 3466, with an average density of 3.07 ± 0.66 barnacles cm−2 (mean ± SE). Mean barnacle basal diameters ranged from 0.29 to 0.42 cm and apart from one quadrat showed very low local variance, suggesting that recruitment was near-simultaneous. Detailed summaries of barnacle size, density and spatial pattern characteristics are provided in Table 1.
Pair correlation functions invariably found evidence of clustering at scales starting from 0.1 cm and up to 5.0 cm [see Fig. 3 for an example; for all other plots see Fig. S1 in Electronic Supplementary Material (ESM)]. The peak of the g(r) function indicates the distance from any given barnacle at which the presence of another barnacle is most likely. This was never more than 0.45 cm, with a mean of 0.36 ± 0.02 cm. This is consistent with the hypothesis that barnacles prefer to remain within 2.5 cm of one another for reproductive access, whilst also indicating a much smaller-scale aggregative force. Mean barnacle diameter was 0.35 ± 0.02 cm. This implies that barnacles tend to establish at twice the mean adult radius, either from existing barnacles or from other settlers. The crowding coefficient C indicates that there is great variation in the degree of direct contact between barnacles (0.13–3.42 among quadrats).
a Pattern of barnacles in a single 20 × 20 cm quadrat (Site 1, Quadrat a). b Spatial point pattern analysis of barnacles in (a). Empirical pair correlation function (g(r), solid line) assessed against the fifth highest and lowest of 999 simulations of a null model of random toroidal transformation of live barnacles relative to dead (\(\alpha =0.01\); simulation envelope in gray with model mean marked as dashed line). See Fig. S1 in ESM for all plot maps and analyses
Close proximity is likely to lead to competition for space and resources, and thereby to subsequent mortality. Cross-pair correlation plots revealed that living individuals are more clustered around dead individuals than expected by chance at points between 0.1 and 1.5 cm (see Fig. 4 for an example; for all other plots see Fig. S2 in ESM). A qualitatively identical pattern was observed in all five quadrats for which sufficient data were available; notably the remaining plots had low crowding coefficients (\(C < 1\)), indicative of limited physical contact between barnacles. The mean distance to the peak probability of finding a live barnacle from any given dead individual was 0.34 ± 0.04 cm. This shows a remarkable correspondence with the pattern in the population as a whole, and demonstrates that dead barnacles had an even greater number of competitors at this distance than would be expected given the existing pattern structure.
Cross-pair correlation function for living relative to dead barnacles in a single 20 × 20 cm quadrat (Site 1, Quadrat a) Empirical pair correlation function (\(g_{ij}(r)\), solid) assessed against the fifth highest and lowest of 999 simulations of a null model of random toroidal transformation of live barnacles relative to dead (\(\alpha =0.01\); simulation envelope in gray with model mean marked as dashed line). See Fig. S2 in ESM for all plot maps and analyses
Discussion
Our analysis of spatial patterning in S. balanoides extends previous work suggesting that individuals cluster at scales below 2.5 cm in order to maintain reproductive access (Kent et al. 2003) by demonstrating a preferred distance of only 0.36 cm. This is much closer than required given the constraints of penis length and implies an additional set of small-scale attractive forces generating aggregations in this species (e.g., Fig. 1). Nevertheless, we also show that there is a cost to aggregation—dead barnacles have a higher than expected number of conspecifics at this distance given the existing pattern structure.
The most frequent distance between barnacles of 0.36 cm corresponds to the mean barnacle diameter of 0.35 cm. This suggests that barnacles tend to establish two average adult radii apart. The sharp fall in numbers of neighbours below this distance suggests that this marks the boundary of the zone of repulsion, and likely the end result of competition-mediated mortality. Most other studies have also detected short-range repulsion among barnacles (e.g., Connell 1961; Crisp 1961; Munroe and Noda 2009), but not the subsequent spike in relative density. The intriguing implication is that the benefits of proximity are greatest just at the point where two barnacles come into direct contact, and that larvae self-organise to create this pattern. Possible benefits include structural facilitation as clustered individuals invest 2–5 times less in shell thickness than solitary ones (Bertness et al. 1998). Clustered individuals spend less time hiding from predators and thus more time feeding, and have reduced individual risk of predation (Mauck and Harkless 2001). Clustering also increases thermal buffering, meaning individuals are less likely to die from desiccation (Bertness 1989). These benefits likely outweigh the costs of clustering when barnacles are separated by at least 0.35 cm.
Newly-settled cyprid larvae have a fast shell growth rate compared with established adults (Barnes and Powell 1953) such that, once metamorphosed, their shells expand quickly to fill the available space. It is unlikely that larvae choose to settle at fixed distances apart because barnacles with more space continue to expand in size (Crisp 1960; Leslie 2003). It is more plausible to suppose that density-dependent thinning occurs, and adult size is constrained by the presence of surrounding individuals. This heightens the interest in discovering why larvae choose to settle so close to conspecifics, given that by doing so they limit their own potential size, which in turn will decrease average fecundity (Leslie 2003). Clustering in S. balanoides also generates costs through intraspecific competition for space (Connell 1961; Wu 1980) leading to a higher mortality rate observed in high versus low density populations (42.0 to 8.5 %) caused by crushing and basal-membrane displacement (Hills and Thomason 2003).
Previous research has focussed on the tendency of S. balanoides to site themselves within 2.5 cm of one another, as this is their maximum penis length (Kent et al. 2003) and the species is obligately cross-fertilising (Barnes and Crisp 1956). While the scale of clustering exceeds 2.5 cm in a number of sites (Table 1), this simply indicates that total aggregations exceed this scale, and in a cluster of 5 cm across, the barnacles in the centre should in principle be able to reach (and be reached by) all other barnacles. By contrast, in the facultatively self-fertilising barnacle Chthamalus montagui, which has a similar penis length, larvae are significantly less likely to settle within 2.5 cm of another individual (Kent et al. 2003). This suggests that the zone of attraction for barnacles is dependent on reproductive strategy, with obligate cross-fertilisers such as S. balanoides subject to stronger selection for denser clusters due to reproductive constraints. Note that though sperm-casting has been described in some barnacle species (Barazandeh et al. 2013), S. balanoides invests heavily in a long penis (Hock 2008), and evidence from other acorn barnacles suggests that physical mating is necessary for fertilisation to occur (Kelly et al. 2012; Barazandeh et al. 2013). Even if some sperm-casting occurs, this would only increase our confidence that factors other than reproduction are driving the closely-packed aggregations in this species.
When densities become very high, larvae switch from seeking conspecifics to seeking free space (Kent et al. 2003). We predict that this switching point will occur when settling larvae must actively seek space which is not within another individual’s zone of repulsion. Leslie (2003) considered a density of 0.81–3.40 barnacles cm−2 to be intermediate, with mortality rates increasing below and fecundity falling above this range. All but two of the quadrats in our study had densities within this range, and even the highest (7.51 barnacles cm−2) was roughly half the density defined as ‘high’ by Leslie (2003). Since intermediate density results in the highest average fitness, we presume that the patterning we observed maximises individual fitness payoff. If this is the case, we would expect larvae to start seeking free space when population density is so high that they can no longer settle 0.36 cm apart.
Strong evidence of the costs of close aggregation comes from analyses of post-settlement mortality among established adults. The cross-pair correlation function showed that living individuals clustered around dead individuals from 0.1–1.0 cm, with mean distance to peak density at 0.34 ± 0.04 cm. This crowding exceeds expectations based on null models which incorporate the existing pattern of live individuals and confirms a powerful role for intraspecific mortality in post-settlement mortality, as has been noted in population-scale studies (Jenkins et al. 2008), and hence in the subsequent development of spatial patterns within barnacle populations.
Deevey (1947) calculated a coefficient of crowding C using S. balanoides population data collected by Hatton (1938) to assess the relationship between population density and mortality risk. Mortality rate markedly decreased in populations where individuals had on average ≤1 contact point with neighbours. In the current study, crowding coefficients of study sites with post-settlement mortality data ranged from 1.45–3.42 contacts, and no sites had a C below 1, consistent with intraspecific competition as the most likely cause of their mortality (Table 1). Note that all sites for which insufficient numbers of dead individuals were available for analysis had C values below 1, consistent with Deevey (1947) and providing further implicit confirmation of the importance of competition in mortality. It is unknown how long shells of dead individuals remain on substrata, and it is possible that shells surrounded by other individuals are better protected from damage caused by waves or other abiotic factors (Hui and Moyse 1987; Bertness 1989). If isolated shells are more likely to die as a result of abiotic stress and predation (Connell 1961; Bertness 1989; Kent et al. 2003), it is possible that these mortality events are under-represented in our study, and long-term monitoring of mapped individuals will be required to assess this.
Our study could not detect establishment mortality; this is a crucial process as newly metamorphosed larvae have a 38.0 % chance of dying in the first day (Gosselin and Qian 1996). After this mortality risk reduces greatly; the next 43 days carry a cumulative mortality risk of just 2.1 %. Data collection for our study was carried out in early winter, around four to five months after larval settlement occurred. Our study therefore analysed established adult post-settlement mortality patterns, an addition to existing research given that most studies focus on the patterning and mortality of settling larvae and newly settled adults (e.g., Leslie 2003; Munroe and Noda 2009). Settlement patterns may not give an accurate indication of the implications for final pattern formation since only 0.3 % of settlers successfully recruit as adults (Pineda et al. 2006).
We deliberately selected sites with flat, homogeneous topography to minimise confounding effects. It is nevertheless likely that microtopography or undetected environmental heterogeneity also influenced the settlement and survival of barnacles, potentially interacting with environmental gradients (e.g., Johnson et al. 1998; Munroe et al. 2010). There is however no reason to believe that this will have introduced any systematic bias into our findings. Interspecific competition has also been found to affect S. balanoides spatial patterns (Hui and Moyse 1987). Further work might build in these additional factors to better understand the emergent properties of barnacle aggregations.
Conclusions
Through detailed spatial point pattern analyses we have extended previous work by revealing that barnacles are strongly clustered with peak densities at 0.36 cm apart, almost exactly the distance at which two barnacles touch, and much closer than is required solely for reproductive access. This implies that the benefits of close proximity outweigh the costs of interspecific competition right up to the point at which physical contact occurs. The costs are clear from evidence that dead barnacles have a higher than expected density of live neighbours. The existence of a short-scale zone of repulsion and larger zone of attraction are consistent with the model of self-organisation in aggregations presented by (Couzin and Krause 2003) and widely observed in motile vertebrate species (Fig. 1). These findings demonstrate the value of barnacles as model organisms for the investigation of self-organisation among sessile organisms.
References
Baddeley A, Turner R (2005) Spatstat: an R package for analysing spatial point patterns. J Stat Software 12:1–42
Barazandeh M, Davis CS, Neufeld CJ, Coltman DW, Palmer AR (2013) Something Darwin didn’t know about barnacles: spermcast mating in a common stalked species. Proc Royal Soc B Biol Sci 280(20122):919
Barnes H, Crisp DJ (1956) Evidence of self-fertilisation in certain species of barnacles. J Marine Biol Assoc UK 35:631–639
Barnes H, Powell HT (1953) The growth of Balanus balanoides and Balanus crenatus under varying conditions of submersion. J Marine Biol Assoc UK 32:107–127
Bertness MD (1989) Intraspecific competition and facilitation in a northern acorn barnacle population. Ecology 70:257–268
Bertness MD, Gaines SD, Bermudez D, Sanford E (1991) Extreme spatial variation in the growth and reproductive output of the acorn barnacle Semibalanus balanoides. Marine Ecol Prog Series 75:91–100
Bertness MD, Gaines SD, Yeh SM (1998) Making mountains out of barnacles: the dynamics of acorn barnacle hummocking. Ecology 79:1382–1394
Charnov EL (1980) Sex allocation and local mate competition in barnacles. Marine Biol Lett 1:269–272
Connell JH (1961) Effects of competition, predation by Thais lapillus, and other factors on natural populations of the barnacle Balanus balanoides. Ecol Monog 31:61–104
Couzin ID, Krause J (2003) Self-organization and collective behavior in vertebrates. Adv Study Behav 32:1–75
Couzin ID, Krause J, James R, Ruxton GD, Franks NR (2002) Collective memory and spatial sorting in animal groups. J Theor Biol 218:1–11
Crisp DJ (1960) Factors influencing growth rate in Balanus balanoides. J Animal Ecol 29:95–116
Crisp DJ (1961) Territorial behaviour in barnacle settlement. J Exp Biol 38:429–446
de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI (2002) Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419:899–903
Deevey ES (1947) Life tables for natural populations of animals. Quart Rev Biol 22:283–314
Giardina I (2008) Collective behavior in animal groups: theoretical models and empirical studies. HFSP J 2:205–219
Gosselin LA, Qian PY (1996) Early post-settlement mortality of an intertidal barnacle: a critical period for survival. Marine Ecol Prog Series 135:69–75
Hatton H (1938) Essais de binomie explicative sur quelques especes intercotidales d’algues et d’animaux. Ann de l’Inst Océanograph 17:241–348 (in French)
Hills JM, Thomason JC (2003) Recruitment density can determine adult morphology and fecundity in the barnacle, Semibalanus balanoides. Biofouling 19:205–213
Hock JM (2008) Variation in penis morphology and mating ability in the acorn barnacle, Semibalanus balanoides. J Exp Marine Biol Ecol 359:126–130
Hui E, Moyse J (1987) Settlement patterns and competition for space. In: Southward AJ (ed) Barnacle Biol. Balkema, A. A, pp 363–376
Illian J, Penttinen A, Stoyan H, Stoyan D (2008) Statistical analysis and modelling of spatial point patterns. Wiley
Jenkins SR, Åberg P, Cervin G, Coleman RA, Delany J, Della Santina P, Hartnoll RG (2000) Spatial and temporal variation in settlement and recruitment of the intertidal barnacle Semibalanus balanoides (Crustacea: Cirripedia) over a European scale. J Exp Marine Biol Ecol 243:209–225
Jenkins SR, Murua J, Burrows MT (2008) Temporal changes in the strength of density-dependent mortality and growth in intertidal barnacles. J Animal Ecol 77:573–584
Johnson MP, Hughes RN, Burrows MT, Hawkins JS (1998) Beyond the predation halo: small scale gradients in barnacle populations affected by the relative refuge value of crevices. J Exp Marine Biol Ecol 231:163–170
Kelly MW, Grosberg RK, Sanford E (2012) Love the one you’re with: proximity determines paternity success in the barnacle Tetraclita rubescens. Mol Ecol 21:5088–5098
Kent A, Hawkins JS, Doncaster CP (2003) Population consequences of mutual attraction between settling and adult barnacles. J Animal Ecol 72:941–952
Law R, Illian J, Burslem DFRP, Gratzer G, Gunatilleke CVS, Gunatilleke IAUN (2009) Ecological information from spatial patterns of plants: insights from point process theory. J Ecol 97:616–628
Leslie HM (2003) Positive intraspecific effects trump negative effects in high-density barnacle aggregations. Ecology 86:2716–2725
Mauck RA, Harkless KC (2001) The effect of group membership on hiding behaviour in the northern rock barnacle Semibalanus balanoides. Animal Behav 62:743–748
Munroe DM, Noda T (2009) Spatial pattern of rocky intertidal barnacle recruitment: comparison over multiple tidal levels and years. J Marine Biol Assoc UK 89:345–353
Munroe DM, Noda T, Ikeda T (2010) Shore level differences in barnacle (Chthamalus dalli) recruitment relative to rock surface topography. J Exp Marine Biol Ecol 392:188–192
Nathan R, Muller-Landau H (2000) Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol Evol 15:278–285
Parrish JK, Edelstein-Keshet L (1999) Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284:99–101
Parrish JK, Viscido SV, Grünbaum D (2002) Self-organized fish schools: an examination of emergent properties. Biol Bull 202:296–305
Pawlik JR (1992) Chemical ecology of the settlement of benthic marine invertebrates. Oceanography and Marine Biology - An Annual. Review 30:273–335
Pineda J, Starczak V, Stueckle TA (2006) Timing of successful settlement: demonstration of a recruitment window in the barnacle Semibalanus balanoides. Marine Ecol Prog Series 320:233–237
R Development Core Team (2015) R: a language and environment for statistical computing. URL https://www.r-project.org/
Rasband WS (1997) ImageJ. URL http://imagej.nih.gov/ij/
Ripley BD (1977) Modelling spatial patterns. J Royal Stat Soc Series B (Stat Methodol) 39:172–212
Satumanatpan S, Keough MJ (2001) Roles of larval supply and behavior in determining settlement of barnacles in a temperate mangrove forest. J Exp Marine Biol Ecol 260:133–153
Southward AJ (ed) (1987) Barnacle Biol. Balkema, A. A
Thévanez P (2008) Point Picker URL http://bigwww.epfl.ch/thevenaz/pointpicker/
Watt AS (1947) Pattern and process in the plant community. J Ecol 35:1–22
Wethey DS (1983) Intrapopulation variation in growth of sessile organisms: natural populations of the intertidal barnacle Balanus balanoides. Oikos 40:14–23
Wethey DS (1984) Effects of crowding on fecundity in barnacles: Semibalanus (Balanus) balanoides, Balanus glandula, and Chthamalus dalli. Canadian J Zool 62:1788–1795
Wiegand T, Moloney KA (2004) Rings, circles and null-models for point pattern analysis in ecology. Oikos 104:209–229
Wu RSS (1980) Effects of crowding on the energetics of the barnacle Balanus glandula. Canadian J Zool 58:559–566
Acknowledgments
Peter and Vanessa Hooper provided assistance with data collection. Jon Moore prepared Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hooper, R.C., Eichhorn, M.P. Too close for comfort: spatial patterns in acorn barnacle populations. Popul Ecol 58, 231–239 (2016). https://doi.org/10.1007/s10144-016-0542-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-016-0542-5