Abstract
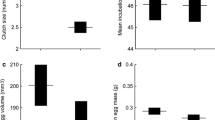
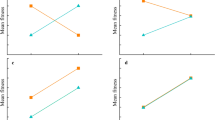
Geographic variation in body size is of special interest because it affects nearly all aspects of an organism’s life. I examined whether differences in body size among four populations of the green anole lizard, Anolis carolinensis, were attributable to maternal investment in egg size and/or growth rates of embryos and juveniles. Larger body size and larger egg size relative to female size in the northern part of the range have been documented in this species, and suggested to be adaptive responses to more extreme winters. The current study confirmed the trends in adult size and egg size in the north, but rejected the trend of larger egg size relative to body size in the south. To control for differences in maternal investment in egg size among populations, I performed yolk removals on eggs from two northern populations to produce comparably sized eggs relative to one southern population. This manipulation was designed to minimize the confounding effect of maternal investment in yolk, the primary energy reserves for eggs, so that intrinsic differences in embryonic growth due to metabolism could be investigated. I found that differences in juvenile and, potentially, embryonic growth rates existed among populations of A. carolinensis, both due to and independent of differences in egg size. Juveniles from the northernmost population were bigger not only due to larger egg size, but also due to faster juvenile growth and possibly differences in developmental stage of oviposition or conversion of egg mass to hatchling mass. Larger body size may hold a number of advantages in northern populations of this species, including starvation resistance through winters and better competitive access to food resources and warmer microhabitats.






Similar content being viewed by others
References
Andrews RM, Mathies T (2000) Natural history of reptilian development: physiological constraints on the evolution of viviparity. Bioscience 50:227–238
Andrews RM, Mathies T, Warner DA (2000) Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol Monogr 2000:420–431
Arnett AE, Gotelli NJ (2003) Bergmann’s rule in larval ant lions: testing the starvation resistance hypothesis. Ecol Entomol 28:645–650
Ashton KG, Feldman CR (2003) Bergmann’s rule in nonavian reptiles: turtles follow it, lizards and snakes reverse it. Evolution 57:1151–1163
Bergmann C (1847) Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Stud 3:595–708 (in German)
Bishop DC (2000) Aspects of the winter behavioral ecology of Anolis carolinensis at the northern limit of its range. MS Thesis, University of Tennessee, Knoxville
Bishop DC, Echternacht AC (2004) Emergence behavior and movements of winter-aggregated green anoles (Anolis carolinensis) and the thermal characteristics of their crevices in Tennessee. Herpetologica 60:168–177
Blackburn TM, Gaston KJ, Loder N (1999) Geographic gradients in body size: a clarification of Bergmann’s rule. Divers Distrib 5:165–174
Brown CR, Brown MB (1998) Intense natural selection on body size and wing and tail asymmetry in cliff swallows during severe weather. Evolution 52:1461–1475
Dessauer HC (1955) Seasonal changes in the gross organ composition of the lizard, Anolis carolinensis. J Exp Zool 128:1–12
Ferguson GW, Fox SF (1984) Annual variation of survival advantage of large juvenile side-blotched lizards, Uta stansburiana: its causes and evolutionary significance. Evolution 38:342–349
Gordon RE (1956) The biology and biodemography of Anolis carolinensis carolinensis Voigt. Ph.D. dissertation, Tulane University, New Orleans, Louisiana
Greenberg DS, Gist DH (1985) Fat bodies and reproduction in female Anolis carolinensis. J Exp Zool 33:277–283
Greenberg B, Noble GK (1944) Social behavior of the American chameleon (Anolis carolinensis Voigt). Physiol Zool 17:392–439
Jenssen TA, Nunez SC (1998) Spatial and breeding relationships of the lizard, Anolis carolinensis: evidence of intrasexual selection. Behaviour 135:981–1003
Jenssen TA, Congdon JD, Fischer RU, Estes R, Kling D, Edmands S, Berna H (1996) Behavioural, thermal, and metabolic characteristics of a wintering lizard (Anolis carolinensis) from South Carolina. Funct Ecol 10:201–209
Ji X, Du WG, Xu WQ (1999) Experimental manipulation of egg size and hatchling size in the cobra, Naja naja atra (Elapidae). Netherlands J Zool 49:167–175
King FW (1966) Competition between two south Florida lizards of the genus Anolis. Ph.D. dissertation, University of Miami, Coral Gables, Florida
Licht P (1973) Influence of temperature and photoperiod on annual ovarian cycle in lizard Anolis carolinensis. Copeia 1973:465–472
McNab BK (1971) On the ecological significance of Bergmann’s rule. Ecology 52:845–854
Meiri S, Dayan T (2003) On the validity of Bergmann’s rule. J Biogeogr 30:331–351
Michaud EJ (1990) Geographic variation of life history traits in the lizard Anolis carolinensis. Ph.D. dissertation, University of Tennessee, Knoxville
Michaud EJ, Echternacht AC (1995) Geographic variation in the life history of the lizard Anolis carolinensis and support for the pelvic constraint model. J Herpetol 29:86–97
Nussey DH, Wilson AJ, Brommer JE (2007) The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Biol 20:831–844
Oufiero CE, Angilletta MJ (2006) Convergent evolution of embyronic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60:1066–1075
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244
Radder RS, Shanbhag BA, Saidapur SK (2004) Yolk partitioning in embryos of the lizard, Calotes vorsicolor: maximize body size or save energy for later use? J Exp Zool 301A:783–785
Rensch B (1938) Some problems of geographical variation and species formation. Proc Linn Soc Lond 150:275–285
Scholander PF (1955) Evolution of climatic adaptation in homeotherms. Evolution 9:15–26
Schultz ET, Conover DO (1997) Latitudinal differences in somatic energy storage: adaptive responses to seasonality in an estuarine fish (Atherinidae: Menidia menidia). Oecologia 109:516–529
Schultz ET, Conover DO (1999) The allometry of energy reserve depletion: test of a mechanism for size-dependent winter mortality. Oecologia 119:474–483
Sinervo B (1990) The evolution of maternal investment in lizards: an experimental and comparative analysis of egg size and its effects on offspring performance. Evolution 44:279–294
Sinervo B, Doughty P (1996) Interactive effects of offspring size and timing of reproduction on offspring reproduction: experimental, maternal, and quantitative genetic aspects. Evolution 50:1314–1327
Sinervo B, Huey RB (1990) Allometric engineering: an experimental test of the causes of interpopulational differences in performance. Science 248:1106–1109
Sinervo B, McEdward LR (1988) Developmental consequences of an evolutionary change in egg size: an experimental test. Evolution 42:885–899
Stamps JA (1984) Rank-dependent compromises between growth and predator protection in lizard dominance hierarchies. Anim Behav 32:1101–1107
Stevenson RD (1985) Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am Nat 125:102–117
Viets BE (1993) Lizard reproductive ecology: sex determination and parental investment. Ph.D. dissertation, Indiana Univeristy, Bloomington
Vitt LJ (2000) Ecological consequences of body size in neonatal and small-bodied lizards in the Neotropics. Herpetol Monogr 14:388–400
Warner DA, Shine R (2007) Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154:65–73
Acknowledgments
I thank A. Echternacht for assistance during this project and comments on this manuscript. J. Fordyce also provided suggestions that greatly improved this manuscript. Thanks to P. Heah, J. Nolt, N. Wyszynski, J. Walguarnery, and A. Fuller, who helped collect data and care for animals in the laboratory. I am grateful to the Department of Ecology and Evolutionary Biology at the University of Tennessee, Knoxville for providing funding and space for this project. Animals in this study were collected under Tennessee Wildlife Resources Agency Scientific Collecting Permit # 1946 and Georgia Department of Natural Resources Scientific Collecting Permit # 29-WSF-05-77. All methods used in this project were approved under the University of Tennessee Institutional Animal Care and Use Committee protocol # 1064.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goodman, R.M. Evidence of divergent growth rates among populations of the lizard Anolis carolinensis based on experimental manipulations of egg size. Popul Ecol 52, 113–122 (2010). https://doi.org/10.1007/s10144-009-0167-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-009-0167-z