Abstract
Spinal dural arteriovenous fistulas (SDAVFs) constitute the most common type of spinal vascular malformations. Their diagnosis requires spinal digital subtraction angiography (DSA), which is time-consuming, requires catheterizing many vessels, and exposes patient to a high radiation and contrast doses. This study aims to evaluate the usefulness of time-resolved MR angiography (TR–MRA) in SDAVF diagnosis. We performed a systematic review of the PubMed and EMBASE databases followed by a meta-analysis. TR–MRA was an index test, and spinal DSA was a reference. Of the initial 324 records, we included 4 studies describing 71 patients with SDAVFs. In 42 cases, TR–MRA was true positive, and in 21 cases, it was true negative. We found 7 false-positive cases and 1 false negative. TR–MRA allowed for shunt level identification in 39 cases. Of these, the predicted level was correct in 23 cases (59%), to within 1 level in 38 cases (97.4%) and to within 2 levels in 39 cases (100%). The diagnostic odds ratio was 72.73 (95% CI [10.30; 513.35]), z = 4.30, p value < 0.0001. The pooled sensitivity was 0.98 (95% CI [0.64; 1.00]), and the pooled specificity was 0.79 (95% CI [0.10; 0.99]). The AUC of the SROC curve was 0.9. TR–MRA may serve as a preliminary study to detect SDAVFs and localize the shunt level with sensitivity and specificity as high as 98% and 79%, respectively. Unless the TR–MRA result is unequivocal, it should be followed by a limited spinal DSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal dural arteriovenous fistulas (SDAVFs) constitute the most common type of spinal vascular malformations. They present with nonspecific symptoms and lead to paraplegia and sphincter dysfunction. Successful treatment may reverse such disastrous symptoms—hence, the need for prompt diagnosis [1, 2].
The diagnosis of SDAVF requires spinal digital subtraction angiography (DSA). Spinal DSA is a time-consuming study that requires catheterizing many vessels, exposes the patient to high radiation and contrast doses, but remains the gold standard. Recently, some researchers reported time-resolved magnetic resonance angiography (TR–MRA) as a useful tool in SDAVF diagnosis.
Time-resolved magnetic resonance angiography is a relatively new MR technique (introduced by Schoenberg et al. in 1999 [3]) which is able to obtain images of vessels in subsequent phases of enhancement after contrast agent administration and thus provide hemodynamic information by visualizing the passage of the contrast through arterial and venous vessels. Advanced MR systems enable the shortening of the acquisition time of each phase to as low as 1–2 s while increasing the temporal resolution. Thus, this enables the imaging of arterial inflow without overlapping veins, which is especially important in the case of such pathologies as SDAVF, in which the inflow into the venous vessels is rapid. However, TR–MRA’s sensitivity, specificity, and accuracy in locating shunt remain unexamined.
This study aims to evaluate the usefulness of TR–MRA in the diagnosis of SDAVF by determining its sensitivity, specificity, and accuracy in locating fistulas. Until now, no one has addressed this question in a systematic review. The significance of this research lies in the fact that such a noninvasive study could improve the diagnostic process of SDAVFs and provide an additional tool in cases of unexplained myelopathy. Furthermore, an initial TR–MRA could greatly facilitate a subsequent spinal DSA by reducing the number of catheterized vessels, as well as the radiation and contrast dose.
Methods
Search strategy and selection criteria
This review follows the PRISMA 2020 guidelines for systematic reviews [4]. We designed and conducted a comprehensive literature search of the PubMed and EMBASE databases. We looked for eligible studies from the inception of each database to October 2022 based on the following phrases: (spinal dural arteriovenous fistula) AND (“Magnetic Resonance Angiography”[Mesh]) for PubMed and analogical for EMBASE; we did not use additional filters.
Our diagnosis of interest was dorsal intradural spinal vascular malformation according to the Spetzler classification [5]. TR–MRA was an index test and spinal DSA was a reference. In selected cases, we considered unequivocal intraoperative findings (and not a spinal DSA) as conformation of SDAVF. When clinical outcomes were evident for the absence of a SDAVF, we characterized such cases as true negatives.
We included studies published in English that reported patients with a suspicion of SDAVF that was based on clinical and radiological evaluation. SDAVFs have characteristic appearance in standard contrast-enhanced spinal MRI: hyperintense signal of spinal cord in T2 sequence and intradural dilated and tortuous vessels [6, 7]. We excluded studies performed in populations with already confirmed SDAVFs (when SDAVF has been diagnosed and TR–MRA was used afterwards, e.g., for research purposes or to gain experience in TR–MRA). We also excluded studies that disregarded TR–MRA results in patients with initial suspicion of SDAVF that was subsequently excluded in DSA. In short, our inclusion and exclusion criteria were as follows:
-
Papers that reported patients with a clinical or radiological suspicion of SDAVF, in which TR–MRA was performed first, and then spinal DSA (or surgery) served as confirmatory tests.
-
Papers that failed to report the results of both studies (TR–MRA and spinal DSA) in each patient.
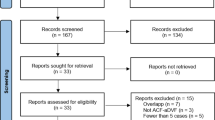
Two independent reviewers screened 272 titles and abstracts and then read the full text of the remaining 21 articles. Seven articles were excluded because the authors reported their experience in SDAVF diagnosis with noninvasive imaging techniques; those were not studies that evaluated population with SDAVF suspicion. We excluded 8 further studies because different imaging modalities were used. Two other studies initially had seemed to have met the inclusion criteria but were later excluded as described later in the text. Disagreements were resolved by seeking the opinion of the experienced neuroradiologist (Fig. 1).
Data extraction
We designed a data extraction form that was filled by two reviewers for each accepted study, and it included population, study design (randomized or nonrandomized, prospective or retrospective), year of publication, number of patients, number of nondiagnostic TR–MRA exams, sensitivity, specificity, positive and negative predictive values, and accuracy in locating fistula level (Table 1). Table 2 presents selected TR–MRA parameters [magnetic field strength of MR scanner, repetition time (TR), echo time (TE), flip angle, FOV, type and dose of contrast, temporal update, slice thickness, and number of postcontrast phases]. The experienced neuroradiologist evaluated the TR–MRA protocols.
The outcomes of this study were TR–MRA sensitivity, specificity, positive and negative predictive values, and accuracy in locating shun level in SDAVF diagnosis. We used the Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS–2) tool for assessing the quality of diagnostic accuracy [8].
Statistical analysis
We estimated from each study means and 95% confidence intervals (CIs) for sensitivity and specificity and presented them with paired forest plots. The Wilson method was used to calculate CI.
The mean age of patients from two of the four analyzed studies was estimated based on median values using a method described by Hozo et al. [9]. We created summary receiver-operating characteristic (SROC) curves with false-positive rate on the x-axis and sensitivity on the y-axis. We used paired forest plots and SROC curves to provide a subjective assessment of the presence or absence of heterogeneity. The bivariate model estimated the summary parameters sensitivity and specificity of the included studies. Continuity correction of 0.5 was used, if applicable. Heterogeneity between studies was assessed using I2 (25–49% was considered to be low heterogeneity, 50–74% was moderate, and > 75% was high heterogeneity). However, as I2 can be biased in small meta-analyses, we have reported the results of both random and fixed models in all comparisons [10].
We did not perform the sensitivity analysis. To conduct meta-analysis, R environment with mada, meta, metafor, and metavix packages was used [11]. We did not use automation tools in our review. We did not need to gather additional information from study investigators with the exception to the studies by Amarouche et al. and Kannath et al. [12, 13]. In these cases, however, with no response, the studies were eventually excluded (as described in detail below).
Results
Literature search results
This systematic review included 4 studies, summarized in Table 1. Risk of bias was assessed with QUADAS–2 tool (On–line Table 1).
In this systematic review, we included 71 patients with distinctive clinical presentation or MR findings suggestive of SDAVFs. Study sizes ranged from 11 to 27 patients. Seventy-six percent (54/71) of patients were male; mean age of patients was 61 years old, range 24–91. We identified 43 cases of SDAVFs, which were diagnosed with spinal DSA (in 41 cases) or intraoperatively (in 2 cases)—when spinal DSA failed to present SDAVF. In the remaining 28 cases, SDAVFs were excluded: in 24 cases with spinal DSA and in 4 cases by clinical observation.
There were performed 71 TR–MRAs and 67 spinal DSAs. In 4 cases with negative TR–MRAs, spinal DSAs were called off. In those cases, the researchers excluded SDAVFs based on medical history, presentation, and clinical course that were unequivocal for lack of spinal vascular malformation.
All imaging studies considered in this systematic review were performed on 1.5-T MR scanners. Table 2 summarizes the other parameters of TR–MRA techniques.
In 42 cases, TR–MRA was true positive, and in 21 cases, it was true negative. We found 7 false-positive cases and 1 false negative (On–line Table 2).
TR–MRA allowed for shunt level identification in 39 cases in total. Of these, the predicted level was correct in 23 cases (59%), to within 1 level in 38 cases (97.4%) and to within 2 levels in 39 cases (100%).
Meta-analysis results
Four studies reported diagnostic accuracy and provided contingency tables with raw diagnostic accuracy of TR–MRA in SDAVF. The diagnostic odds ratio was 72.73 (95% CI [10.30; 513.35]), z = 4.30, p value < 0.0001 (Fig. 2). The funnel plot shows publication bias (Fig. 3). The pooled sensitivity was 0.98 (95% CI [0.64; 1.00]) (Fig. 4A), and the pooled specificity was 0.79 (95% CI [0.10; 0.99]) (Fig. 4B). The area under curve (AUC) of the summary receiver-operating characteristic (SROC) curve was 0.9 (Fig. 5).
Summary receiver-operating characteristic (SROC) curve showing the relationship between the false-positive rate on the x-axis and sensitivity on the y-axis. The point estimate of the pair of sensitivity and false-positive rate is shown by a black, open circle. The red, dashed line represents the 95% confidence region. Blue triangles represent input data (results from particular studies)
Description of included studies
Ali et al. used 1.5-T TR–MRA and spinal DSA to evaluate 11 patients with suspected spinal vascular malformations, 3 of which were SDAVFs [14]. The researchers initially divided patients into high and low suspicion, which was based on presence or absence of early venous shunting in TR–MRA. They inferred level of the shunt through a thorough examination of the TR–MRA. Patients with high suspicion of an arteriovenous fistula underwent spinal DSA, whereas patients with low suspicion either had a spinal DSA or were followed clinically. In cases where TR–MRA was negative, patients were considered true negatives based on clinical outcomes. Subsequent spinal DSAs were planned based on TR–MRA, as described above, with high agreement between the two tests in predicting the location of the shunt. The study reported 100% sensitivity and specificity and no false positives (FPs) or false negatives (FNs).
Saindane et al. evaluated 18 patients using 1.5-T MR, including 8 with SDAVFs [15]. All patients underwent DSA after TR–MRA. Specifically, when TR–MRA was suggestive of a specific arterial feeder, this vessel was catharized first during spinal DSA. When TR–MRA was negative, the spinal DSA was performed in regular fashion. In 4 cases, TR–MRA had limited diagnostic value due to improper scan initiation or patient movements. The sensitivity, specificity, and positive and negative predictive values were 88%, 90%, 88%, and 90%, respectively. TR–MRA matched spinal DSA in 7 cases, with feeding arteries in 6 cases within one vertebral level. In the seventh case, a SDAVF was correctly identified on the TR–MRA, but the feeding artery was not.
Mathur et al. presented 15 patients who had TR–MRA, of which 10 had SDAVF [16]. In all cases, spinal DSA served as a reference test. The researchers first catheterized segmental arteries that were most likely to supply a fistula based on TR–MRA findings. In case the fistula was not identified in such way, the full spinal DSA was performed. The overall sensitivity, specificity, and positive and negative predictive values were 100%, 80%, 91%, and 100%, respectively; all shunt levels were correctly identified to within 1 vertebral level.
Naamani et al. reported 27 patients with SDAVF suspicion, which was subsequently confirmed in 22 cases (20 in spinal DSA and 2 intraoperatively) [17]. In their institution, any patient with an SDAVF suspicion has the spinal DSA following TR–MRA. In 2 cases, the TR–MRA tested positive for SDAVF, contrary to following spinal DSA. Despite that, the vascular malformations were confirmed intraoperatively. Overall sensitivity, specificity, and positive predictive values were 100%, 0%, and 71.4%, while negative predictive value could not be calculated.
Out of the 20 SDAVFs that were positive on both diagnostics tools, 10 (50%) were at the exact level as suggested by the TR–MRA and 10 (50%) were at a different location. With respect to the 10 patients who had discrepancies in their SDAVF localization between the 2 modalities, 9 (90%) patients had a 1-level difference, and 1 (10%) patient had a 2-level difference. Thus, the accuracy of the TR–MRA was 50% in predicting the exact fistula level and 90% accurate in predicting the level correctly to within 1 level.
Description of excluded studies
Two studies by Kannath et al. and by Amarouche et al. initially had seemed to have met the inclusion criteria but were later excluded.
In a study by Kannath et al. (according to the on-line supplemental diagram), 3 patients from the group with suspected spinal vascular malformation were excluded, because their subsequent spinal DSAs were negative [13]. The authors did not enclose TR–MRA results in these cases, which precludes estimating specificity.
A study by Amarouche et al. lacked the information about false-positive TR–MRAs (what kind of SAVM was suspected) and the results of spinal DSA in cases with false-negative TR–MRAs [12]. Thus, estimating sensitivity and specificity is impossible. The authors tried contacting the researchers but with no success.
An illustrative case
A 61-year-old man presented with a 5-year history of progressive paraparesis of the lower limbs and concomitant urinary disturbance. Upon admission, his neurological examination revealed impaired sensation, spastic lower limb paresis, hyperactive tendon reflexes, and a Babinski sign. Conventional spinal MRI exhibited hyperintensity of the spinal cord in T2-weighted sequences and perimedullary flow voids (Fig. 6).
Images of the described illustrative case before (a–d) and after (e, f) surgical treatment. An initial sagittal T2-weighted MR image (a) displays thickened torturous vessels on the posterior surface of the thoracic spinal cord (a, thin arrows) and hyperintensive spinal cord (a, big arrow), indicative of myelopathy: these findings are suggestive of spinal dural arterio-venous fistula (SDAVF). A multiplanar reconstruction (MPR) in the coronal plane of time-resolved MR angiography (TR–MRA, b) reveals a SDAVF, which is consistent with spinal digital subtraction angiography (DSA, c). A maximum intensity projection (MIP) reconstruction in the oblique plane (d) before and a subtracted source image (e) after the surgery demonstrate the resolution of a SDAVF, further highlighted on the volume rendering (VR) reconstruction (f). A circle marks a SDAVF on b–d and its reduction on e, f. MR, magnetic resonance
TR–MRA revealed an early draining and prominent intradural radicular vein, demonstrating a transition from the feeding artery to the arterialized vein at the Th7 level on the left.
This observation was confirmed with spinal DSA. Following the diagnosis of SDAVF, a unilateral hemilaminectomy was performed to surgically disconnect the fistula. Identification and closure of the arterialized vein were achieved, with confirmation provided by intraoperative indocyanine green videoangiography. The patient exhibited favorable tolerance to the procedure. Postoperative TR–MRA did not manifest any residual features indicative of SDAVF. Upon 1-year follow-up, the patient demonstrated significant neurological improvement, achieving unassisted ambulation, albeit with residual minor urinary dysfunction.
Discussion
Accurately identifying and precisely locating SDAVF are prerequisite for swift and successful surgery. Development of a noninvasive diagnostic tool is a promising idea but requires objective evaluation, which was the reason for this study. In our meta-analysis, we demonstrated that TR–MRA might have sensitivity and specificity in SDAVF detection as high as 98% and 79%, respectively. Based on our results, we believe that TR–MRA has earned the status of and could serve as noninvasive screening study for patients with suspected SDAVFs.
Spinal DSA limitations
It is worth emphasizing that even though spinal DSA is—and probably will remain—a gold standard, it may mislead in certain cases. In studies by Naamani et al., Przepiorka et al., and Oldfield et al., spinal DSA failed to present SDAVFs that were confirmed intraoperatively [6, 17, 18].
Advantages and use of TR–MRA
However, there is not enough evidence (nor is the MRI technology advanced enough) to support the notion that TR–MRA should replace spinal DSA. Specifically, it is important to highlight that a negative TR–MRA is not always sufficient to exclude a fistula. Yet, when cautiously interpreted, it may serve as a scout study to facilitate subsequent spinal DSA. As an auxiliary tool, TR–MRA may transform spinal DSA from a long and repetitive diagnostic procedure into a quick confirmatory study with minimal vessels catheterized and reduced iodine contrast and electromagnetic radiation doses.
TR–MRA findings suggestive for SDAVF
The optimal utilization of the TR MRA technique hinges on the execution of a technically precise examination, facilitating the acquisition of high-quality diagnostic images and their accurate interpretation. Addressing the initial concern, the reduction of the acquisition time for each phase to only 1–2 s enhances temporal resolution, allowing for the imaging of arterial inflow without overlapping veins. Additionally, employing the subtraction technique eliminates static background interference, akin to the principles of DSA, thereby enhancing the visualization of contrasted vessels. Secondly, a meticulous phase-by-phase assessment of both source and reconstructed images assumes paramount importance in the overall evaluation of the study.
After reviewing several research papers, particularly the studies conducted by Mathur et al. and Ali et al., the distinct TR–MRA characteristics and techniques for localizing SDAVFs are adopting their descriptions: (1) a smudge of enhancement in the area of the nerve root dural sleeve that is connected to a branch of the segmental artery; (2) a significant, early draining intradural radicular vein; (3) a morphological transition from feeding arteries to arterialized veins; (4) a cine review of anterograde flow features; and (5) thorough analysis and correlation with unsubtracted source images (as elucidated in the preceding exposition) [14, 16].
The phenomenon known as the “missing-piece sign,” initially elucidated by Zalewski et al. in 2018, visible in most cases in the late phase of the examination, offers valuable assistance in pinpointing the fistula site [19]. This sign manifests as a discrete region of nonenhancement within an extended segment characterized by intense gadolinium enhancement of the spinal cord. It is likely attributed to the variability within the intrinsic venous system of the spinal cord. The segments devoid of enhancement may suggest more effective venous outflow routes compared to the neighboring cord [20].
Novel multidisciplinary SDAVF diagnosis and treatment algorithm
Based on the results presented herein, we propose the following SDAVF management algorithm that requires effective cooperation between neurosurgeons, MRI radiologists, and interventional radiologists. Firstly, a neurosurgeon should infer the spinal region (as broad as the cervical, thoracic, or lumbosacral region) suspected of SDAVF based on standard spinal MRI and the patient’s clinical presentation. Secondly, MRI radiologists would plan and execute the TR–MRA to locate (or at least approximate) the fistula location. Based on that, an interventional radiologist would precisely diagnose SDAVF with a limited spinal DSA. Finally, a neurosurgeon would be able to disconnect the fistula (or a neurointerventionalist would embolize it) leading to the patient being cured.
Future directions
Future studies should focus on establishing optimal MRI parameters (such as dose and type of contrast, number of postcontrast phases, temporal update, slice thickness, and features of false-positive results). Additionally, TR–MRA application in other types of spinal vascular malformations, namely, cranial dural arteriovenous fistulas, is yet to be explored.
Limitations
Studies considered in this systematic review are limited by the small number of patients included. What is more, most of the studies used different MRI protocols. Finally, we could not include one of the biggest series published to date as described in detail above.
Similarly, conclusions from our meta-analysis are limited by a low number of studies included (less than five) and sample sizes (less than 30 patients per group) [21].
Conclusions
This systematic review and meta-analysis show that TR–MRA may serve as a preliminary study to detect SDAVFs and localize the shunt level with sensitivity and specificity as high as 98% and 79%, respectively. Unless TR–MRA results are unequivocal, it should be followed by limited spinal DSA to confirm SDAVF diagnosis and precisely locate the shunt level. Future studies are needed to further evaluate TR–MRA and advance its development.
Data availability
Data used in this study will be available upon reasonable request should all authors agree.
References
Brinjikji W, Nasr DM, Morris JM, Rabinstein AA, Lanzino G (2016) Clinical outcomes of patients with delayed diagnosis of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 37(2):380–386. https://doi.org/10.3174/ajnr.A4504
Safaee MM, Clark AJ, Burkhardt JK, Winkler EA, Lawton MT (2018) Timing, severity of deficits, and clinical improvement after surgery for spinal dural arteriovenous fistulas. J Neurosurg Spine 29(1):85–91. https://doi.org/10.3171/2017.11.SPINE17988
Schoenberg SO, Wunsch C, Knopp MV et al (1999) Abdominal aortic aneurysm. Detection of multilevel vascular pathology by time-resolved multiphase 3D gadolinium MR angiography: initial report. Invest Radiol. 34(10):648–659. https://doi.org/10.1097/00004424-199910000-00007
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Spetzler RF, Detwiler PW, Riina HA, Porter RW (2002) Modified classification of spinal cord vascular lesions. J Neurosurg 96(2 Suppl):145–156. https://doi.org/10.3171/spi.2002.96.2.0145
Przepiórka Ł, Kunert P, Juszyńska P et al (2018) Coincidence of tethered cord, filum terminale lipoma, and sacral dural arteriovenous fistula: report of two cases and a literature review. Front Neurol 9:807. https://doi.org/10.3389/fneur.2018.00807
Wojciechowski J, Kunert P, Nowak A et al (2017) Surgical treatment for spinal dural arteriovenous fistulas: outcome, complications and prognostic factors. Neurol Neurochir Pol 51(6):446–453. https://doi.org/10.1016/j.pjnns.2017.07.001
Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
von Hippel PT (2015) The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol 15:35. https://doi.org/10.1186/s12874-015-0024-z
R Core Team (2021) R: a language and environment for statistical computing. Published online. https://www.R-project.org/. Accessed 14 Mar 2023
Amarouche M, Hart JL, Siddiqui A, Hampton T, Walsh DC. Time-resolved contrast-enhanced MR angiography of spinal vascular malformations. American Journal of Neuroradiology. Published online November 13, 2014. https://doi.org/10.3174/ajnr.A4164
Kannath SK, Mandapalu S, Thomas B, Enakshy Rajan J, Kesavadas C (2019) Comparative analysis of volumetric high-resolution heavily T2-weighted MRI and time-resolved contrast-enhanced MRA in the evaluation of spinal vascular malformations. AJNR Am J Neuroradiol 40(9):1601–1606. https://doi.org/10.3174/ajnr.A6164
Ali S, Cashen TA, Carroll TJ et al (2007) Time-resolved spinal MR angiography: initial clinical experience in the evaluation of spinal arteriovenous shunts. AJNR Am J Neuroradiol 28(9):1806–1810. https://doi.org/10.3174/ajnr.A0639
Saindane AM, Boddu SR, Tong FC, Dehkharghani S, Dion JE (2015) Contrast-enhanced time-resolved MRA for pre-angiographic evaluation of suspected spinal dural arterial venous fistulas. J Neurointerv Surg 7(2):135–140. https://doi.org/10.1136/neurintsurg-2013-010981
Mathur S, Bharatha A, Huynh TJ, Aviv RI, Symons SP (2017) Comparison of time-resolved and first-pass contrast-enhanced MR angiography in pretherapeutic evaluation of spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol 38(1):206–212. https://doi.org/10.3174/ajnr.A4962
Naamani KE, Abbas R, Tartaglino L et al (2022) The accuracy of the TRICKS MRI in diagnosing and localizing a spinal dural arteriovenous fistula: a feasibility study. World Neurosurg 158:e592–e597. https://doi.org/10.1016/j.wneu.2021.11.020
Oldfield EH, Bennett A, Chen MY, Doppman JL (2002) Successful management of spinal dural arteriovenous fistulas undetected by arteriography. Report of three cases. J Neurosurg 96(2 Suppl):220–229. https://doi.org/10.3171/spi.2002.96.2.0220
Zalewski NL, Rabinstein AA, Brinjikji W et al (2018) Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol 75(12):1542–1545. https://doi.org/10.1001/jamaneurol.2018.2605
Zhu J, Zhu W, Li M, Wei X, Chen Z, Li Y (2023) MRI characteristics of lumbosacral dural arteriovenous fistulas. Front Neurol 14:1157902. https://doi.org/10.3389/fneur.2023.1157902
Zhou S, Shen C (2022) Avoiding definitive conclusions in meta-analysis of heterogeneous studies with small sample sizes. JAMA Otolaryngol Head Neck Surg 148(11):1003–1004. https://doi.org/10.1001/jamaoto.2022.2847
Author information
Authors and Affiliations
Contributions
Conceptualization, Ł.P. and K.W.; methodology, Ł.P., K.W., and S.K.; software, S.K.; validation, E.M., A.M., and P.K.; formal analysis, Ł.P., K.W., and S.K.; investigation, K.C., Ł.P., and E.M.; resources, K.W.; data curation, E.M. and A.M.; writing—original draft preparation, K.W. and Ł.P.; writing—review and editing, Ł.P. and S.K.; visualization, S.K.; supervision, E.M. and A.M.; project administration, E.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This is an observational study. The Bioethics Committee of the Medical University of Warsaw has confirmed that no ethical approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wójtowicz, K., Przepiorka, L., Maj, E. et al. Usefulness of time-resolved MR angiography in spinal dural arteriovenous fistula (SDAVF)—a systematic review and meta-analysis. Neurosurg Rev 47, 9 (2024). https://doi.org/10.1007/s10143-023-02242-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02242-7