Abstract
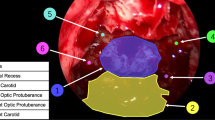
Accurate tumor identification during surgical excision is necessary for neurosurgeons to determine the extent of resection without damaging the surrounding tissues. No conventional technologies have achieved reliable performance for pituitary adenomas. This study proposes a deep learning approach using intraoperative endoscopic images to discriminate pituitary adenomas from non-tumorous tissue inside the sella turcica. Static images were extracted from 50 intraoperative videos of patients with pituitary adenomas. All patients underwent endoscopic transsphenoidal surgery with a 4 K ultrahigh-definition endoscope. The tumor and non-tumorous tissue within the sella turcica were delineated on static images. Using intraoperative images, we developed and validated deep learning models to identify tumorous tissue. Model performance was evaluated using a fivefold per-patient methodology. As a proof-of-concept, the model’s predictions were pathologically cross-referenced with a medical professional’s diagnosis using the intraoperative images of a prospectively enrolled patient. In total, 605 static images were obtained. Among the cropped 117,223 patches, 58,088 were labeled as tumors, while the remaining 59,135 were labeled as non-tumorous tissues. The evaluation of the image dataset revealed that the wide-ResNet model had the highest accuracy of 0.768, with an F1 score of 0.766. A preliminary evaluation on one patient indicated alignment between the ground truth set by neurosurgeons, the model’s predictions, and histopathological findings. Our deep learning algorithm has a positive tumor discrimination performance in intraoperative 4-K endoscopic images in patients with pituitary adenomas.



Similar content being viewed by others
Data availability
The datasets generated during the current study and model codes are available from the corresponding author upon reasonable request.
References
Melmed S, Casanueva FF, Hoffman AR et al (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288. https://doi.org/10.1210/jc.2010-1692
Aghi MK, Chen CC, Fleseriu M et al (2016) Congress of Neurological Surgeons Systematic Review and evidence-based guidelines on the management of patients with nonfunctioning pituitary adenomas: executive summary. Neurosurgery 79:521–523. https://doi.org/10.1227/NEU.0000000000001386
Honegger J, Grimm F (2018) The experience with transsphenoidal surgery and its importance to outcomes. Pituitary 21:545–555. https://doi.org/10.1007/s11102-018-0904-4
Behbahaninia M, Martirosyan NL, Georges J et al (2013) Intraoperative fluorescent imaging of intracranial tumors: a review. Clin Neurol Neurosurg 115:517–528. https://doi.org/10.1016/J.CLINEURO.2013.02.019
Deng J, Dong W, Socher R, Li LJ, Li K, Fei-Fei L (2009) ImageNet: a large-scale hierarchical image database. 2009 IEEE Conference on Computer Vision and Pattern Recognition, pp 248–255. https://doi.org/10.1109/CVPR.2009.5206848
Lui TKL, Guo CG, Leung WK (2020) Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and meta-analysis. Gastrointest Endosc 92:11-22.e6. https://doi.org/10.1016/J.GIE.2020.02.033
Nagata Y, Takeuchi K, Yamamoto T et al (2019) Peel-off resection of the pituitary gland for functional pituitary adenomas: pathological significance and impact on pituitary function. Pituitary 22:507–513. https://doi.org/10.1007/s11102-019-00980-w
Nagata Y, Takeuchi K, Yamamoto T et al (2019) Removal of the medial wall of the cavernous sinus for functional pituitary adenomas: a technical report and pathologic significance. World Neurosurg 126:53–58. https://doi.org/10.1016/J.WNEU.2019.02.134
Ishikawa T, Takeuchi K, Nagata Y et al (2018) Three types of dural suturing for closure of CSF leak after endoscopic transsphenoidal surgery. J Neurosurg 131:1625–1631. https://doi.org/10.3171/2018.4.JNS18366
Giustina A, Chanson P, Bronstein MD et al (2010) A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148. https://doi.org/10.1210/jc.2009-2670
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. 2006 IEEE Conference on Computer Vision and Pattern Recognition, pp. 770–778. https://doi.org/10.1109/CVPR.2016.90
Zagoruyko S, Komodakis N (2016) Wide residual networks. Proceedings of the British Machine Vision Conference, p. 87.1–87.12. https://doi.org/10.5244/C.30.87
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ (2017) Densely connected convolutional networks. 2017 IEEE Conference on Computer Vision and Pattern Recognition, pp. 2261–2269. https://doi.org/10.1109/CVPR.2017.243
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302. https://doi.org/10.2307/1932409
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Munksgaard, Copenhagen
Rolston JD, Han SJ, Aghi MK (2016) Nationwide shift from microscopic to endoscopic transsphenoidal pituitary surgery. Pituitary 19:248–250. https://doi.org/10.1007/s11102-015-0685-y
Nagata Y, Takeuchi K, Yamamoto T, Mizuno A, Wakabayashi T (2020) Fully endoscopic transcylinder trans-magendie foraminal approach for fourth ventricular cavernoma: a technical case report. World Neurosurg 142:104–107. https://doi.org/10.1016/j.wneu.2020.06.171
Nagata Y, Watanabe T, Nagatani T, Takeuchi K, Chu J, Wakabayashi T (2017) The multiscope technique for microvascular decompression. World Neurosurg 103:310–314. https://doi.org/10.1016/j.wneu.2017.04.059
Marcus HJ, Hughes-Hallett A, Cundy TP et al (2014) Comparative effectiveness of 3-dimensional vs 2-dimensional and high-definition vs standard-definition neuroendoscopy: a preclinical randomized crossover study. Neurosurgery 74:375–380. https://doi.org/10.1227/NEU.0000000000000249
Uozumi Y, Taniguchi M, Nakai T, Kimura H, Umehara T, Kohmura E (2020) Comparative evaluation of 3-dimensional high definition and 2-dimensional 4-K ultra-high definition endoscopy systems in endonasal skull base surgery. Oper Neurosurg (Hagerstown) 19:281–287. https://doi.org/10.1093/ons/opz426
Cho SS, Lee JYK (2019) Intraoperative fluorescent visualization of pituitary adenomas. Neurosurg Clin N Am 30:401–412. https://doi.org/10.1016/j.nec.2019.05.002
Akutsu N, Taniguchi M, Kohmura E (2016) Visualization of the normal pituitary gland during the endoscopic endonasal removal of pituitary adenoma by narrow band imaging. Acta Neurochir (Wien) 158:1977–1981. https://doi.org/10.1007/s00701-016-2901-6
Chang SW, Donoho DA, Zada G (2019) Use of optical fluorescence agents during surgery for pituitary adenomas: current state of the field. J Neurooncol 141:585–593. https://doi.org/10.1007/s11060-018-03062-2
Micko A, Rapoport BI, Youngerman BE et al (2020) Limited utility of 5-ALA optical fluorescence in endoscopic endonasal skull base surgery: a multicenter retrospective study. J Neurosurg 1–7. https://doi.org/10.3171/2020.5.JNS201171
Marbacher S, Klinger E, Schwzer L et al (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36:E10. https://doi.org/10.3171/2013.12.FOCUS13464
Vergeer RA, Theunissen REP, van Elk T et al (2022) Fluorescence-guided detection of pituitary neuroendocrine tumor (PitNET) tissue during endoscopic transsphenoidal surgery available agents, their potential, and technical aspects. Rev Endocr Metab Disord 23:647–657. https://doi.org/10.1007/s11154-022-09718-9
Krizhevsky A, Sutskever I, Hinton GE (2017) ImageNet classification with deep convolutional neural networks. Commun ACM 60:84–90. https://doi.org/10.1145/3065386
Spadaccini M, Iannone A, Maselli R et al (2021) Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 6:793–802. https://doi.org/10.1016/S2468-1253(21)00215-6
Sharma P, Hassan C (2022) Artificial intelligence and deep learning for upper gastrointestinal neoplasia. Gastroenterology 162:1056–1066. https://doi.org/10.1053/J.GASTRO.2021.11.040
Repici A, Badalamenti M, Maselli R et al (2020) Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology 159:512-520.e7. https://doi.org/10.1053/J.GASTRO.2020.04.062
Su JR, Li Z, Shao XJ et al (2020) Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc 91:415-424.e4. https://doi.org/10.1016/J.GIE.2019.08.026
Wang P, Liu X, Berzin TM et al (2020) Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol 5:343–351. https://doi.org/10.1016/S2468-1253(19)30411-X
Staartjes VE, Volokitin A, Regli L, Konukoglu E, Serra C (2021) Machine vision for real-time intraoperative anatomic guidance: a proof-of-concept study in endoscopic pituitary surgery. Oper Neurosurg (Hagerstown) 21:242–247. https://doi.org/10.1093/ons/opab187
Rigante M, La Rocca G, Lauretti L et al (2017) Preliminary experience with 4K ultra-high definition endoscope: analysis of pros and cons in skull base surgery. Acta Otorhinolaryngol Ital 37:237–241. https://doi.org/10.14639/0392-100X-1684
Shorten C, Khoshgoftaar TM (2019) A survey on image data augmentation for deep learning. J Big Data 6:60. https://doi.org/10.1186/s40537-019-0197-0
Erhan D, Bengio Y, Courville A, Manzagol PA, Vincent P, Bengio S (2010) Why does unsupervised pretraining help deep learning? J Mach Learn Res 11:625–660
Lee EJ, Ahn JY, Noh T, Kim SH, Kim TS, Kim SH (2009) Tumor tissue identification in the pseudocapsule of pituitary adenoma: should the pseudocapsule be removed for total resection of pituitary adenoma? Neurosurgery 64:ons62–70. https://doi.org/10.1227/01.NEU.0000330406.73157.49
Zhang X, Wang YG, Tan J et al (2022) Comparison of outcomes between intracapsular resection and pseudocapsule-based extracapsular resection for pituitary adenoma: a systematic review and meta-analysis. BMC Neurol 22:52. https://doi.org/10.1186/s12883-022-02574-9
Qu X, Yang J, Sun JD et al (2011) Transsphenoidal pseudocapsule-based extracapsular resection for pituitary adenomas. Acta Neurochir (Wien) 153:799–806. https://doi.org/10.1007/s00701-011-0961-1
Bergman JJGHM, de Groof AJ, Pech O et al (2019) An interactive web-based educational tool improves detection and delineation of Barrett’s esophagus–related neoplasia. Gastroenterology 156:1299-1308.e3. https://doi.org/10.1053/j.gastro.2018.12.021
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9:62–66. https://doi.org/10.1109/TSMC.1979.4310076
Acknowledgements
We would like to thank Editage (www.editage.com) for their editing support on this manuscript.
Funding
This research was funded by the Industry-University Collaborative Project for Human Resource Development to Accelerate AI R&D in the Health and Medical Fields (MEXT).
Author information
Authors and Affiliations
Contributions
Conception and design: Y.F., K.T., and Y.N.; development of methodology: Y.F., K.T., N.H., Y.N., and I.T.; acquisition of data: Y.F. and Y.N.; image annotation: Y.N., K.T., and T.N.; data analysis and deep learning: Y.F., N.H., Y.T., and I.T.; interpretation of data: Y.F., K.T., N.H., Y.N., and I.T.; writing of the initial manuscript: Y.F. and Y.N.; manuscript revision: K.T., N.H., T.N., and I.T.; supervision: I.T. and R.S.; approval of the final manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval
This single-center observational study (2021-0050-2) was approved by our institutional review board. This research was conducted in accordance with the Declaration of Helsinki, as revised in 2013.
Consent to participate
Written informed consent was obtained from all prospectively analyzed patients. Informed consent was obtained from other patients through an opt-out approach on our institution’s website.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fuse, Y., Takeuchi, K., Hashimoto, N. et al. Deep learning based identification of pituitary adenoma on surgical endoscopic images: a pilot study. Neurosurg Rev 46, 291 (2023). https://doi.org/10.1007/s10143-023-02196-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02196-w