Abstract
Chronic subdural hematoma (cSDH) is a common neurosurgical condition that can cause severe morbidity and mortality. cSDH recurs after surgical evacuation in 5–30% of patients, but drains may help reduce this risk. We aimed to investigate the effect of drainage versus no drainage on the rates of recurrence and mortality, as well as the clinical outcomes of cSDH. Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we searched four electronic databases (PubMed, Cochrane Library, Scopus, and Web of Science) to identify eligible studies reported up to June 2022. Using Review Manager software, we reported four primary outcomes as odds ratios (ORs) and confidence intervals (CIs). The meta-analysis included a total of 10 studies with 1961 patients. The use of drainage was found to be significantly more effective than non-drainage in reducing the “mortality rate” (OR = 0.65, 95% CI 0.43 to 0.97; P = 0.04), the “recurrence rate” (OR = 0.39, 95% CI 0.28 to 0.55; P < 0.00001), and occurrence of “gross focal neurological deficit” (OR = 0.58, 95% CI 0.37 to 0.89; P = 0.01). No significant difference was found in the occurrence of a Glasgow Coma Scale score of 15 (OR = 1.21, 95% CI 0.84 to 1.76; P = 0.30). The use of drains after burr-hole irrigation reduces the recurrence, mortality, and gross focal neurological deficit rates of chronic subdural hematomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic subdural hematoma (cSDH) is a long-standing blood clot on the brain’s surface underneath its outer coating [1]. Patients with brain atrophy, or the shrinking or withering away of brain tissue due to age or disease, are most likely to develop these liquid clots when they are 60 years or older [2]. Minor head trauma can break blood vessels over the brain’s surface as the brain shrinks inside the skull over time, leading to a steady blood buildup over several days to weeks [1]. The most frequent complaint is a headache, and symptoms might include weakness, nausea, vomiting, lethargy, disorientation, memory loss, nausea, and seizures [1]. Diagnosis involves computed tomography and magnetic resonance imaging brain scans. SDHs vary in density and may extend over a large portion of the brain’s surface. The best therapy method is burr-hole trepanation, which involves surgically draining the hematoma. The most efficient method of treating cSDH is burr-hole craniostomy, which involves evacuation through one or two burr holes drilled over the location of the hematoma [3]. A significant issue with cSDH is a recurrence, which refers to developing another cSDH in the same location. Patients may require additional surgical interventions to address these recurrent hematomas. The use of drains may reduce the likelihood of recurrence; however, their utilization is not commonly practiced [1].
In addition, middle meningeal artery (MMA) embolization for cSDH has been documented regularly in recent years, and several technological advancements to enhance clinical results have been published [4]. After MMA embolization, it has been discovered that embolic materials that are farther distant help prevent recurrences [4]. Abdollahifard et al. performed a meta-analysis of 11 studies and 359 patients. They reported a pooled recurrence rate of 5% (95% confidence interval (CI) 3 to 8%), a need for reoperation rate of 5% (95% CI 3 to 9%), and a peri-procedural complication rate of 4% (95% CI 2 to 9%) following MMA embolization with particle embolic agents [5]. Khorasanizadeh et al.’s retrospective study involved 78 patients and concluded that using coils for endovascular treatment of cSDHs can be as effective as the adjunct use of particle embolization [6]. Investigating the number of MMA branches embolized showed that embolization of the anterior and posterior MMA branches may be associated with an increased likelihood of complete resolution (76%) compared to single-branch occlusion (33%, P = 0.014) [7].
Research has shown that using a drainage tube significantly reduces recurrence rates compared to treatments without it [8]. A retrospective cohort study of 102 patients with cSDH showed that the risk of mortality and recurrence was 14.5% and 32% for patients undergoing craniotomy compared to only 8.7% and 17.7% of patients receiving burr hole drainage treatment, respectively [8]. Despite the presence of various drainage methods, subdural drainage remains prevalent. A systematic review and meta-analysis of 15 studies involving 4318 patients analyzed different drainage methods affecting postoperative prognosis. They reported insignificant differences between subdural drainage and subperiosteal/subgaleal drainage groups in recurrence rates (odds ratio (OR)= 1.08, 95% CI 0.83 to 1.42), mortality rates (OR = 1.16, 95% CI 0.92 to 1.48), and postoperative infection rates (OR = 1.08, 95% CI 0.60 to 1.95) [9].
Additionally, Peng and Zhu’s meta-analysis sought to determine if using external drains following burr-hole surgery for cSDH lowers the likelihood of the condition returning [10]. When information from new research becomes available, their findings might alter. Even when the data are pooled, the available research contains too few participants or events to provide a valid conclusion. As a result, some of the studies, thought to be of lesser quality, did not fully define the randomization processes.
Therefore, we conduct an updated systematic review and meta-analysis to compare the effects and safety of using external drains following the burr-hole evacuation to treat cSDH in adults. We compared external subdural drains with no drains following a burr-hole evacuation in randomized controlled trials (RCTs) to manage cSDH in adults.
Methods
We followed PRISMA statement guidelines when reporting this systemic review and meta-analysis [11]. All steps were done in accordance with the Cochrane Handbook of Systematic Review and Meta-analysis of Interventions (version 5.1.0) [12].
Eligibility criteria
We included studies in our review if they satisfied the following criteria:
-
(1)
Population: patients with subdural hematoma
-
(2)
Intervention: drain
-
(3)
Comparator: no drain
-
(4)
Outcome:
-
i)
Primary outcomes: mortality and recurrence
-
ii)
Secondary outcomes: hospital stay, neurological deficits, and GCS scale
-
(5)
Study design: we included clinical trials, randomized clinical trials, and observational studies (case–control or cohort studies) that are English and involved at least ≥ 10 human patients with subdural hematoma who had drain or no drain operation
We excluded reviews, case reports, editorial letters, conference abstracts, study protocols, animal and phantom studies, and patients who had other treatments before the mentioned operation.
Search strategy
We searched the following electronic medical databases: PubMed, Scopus, Web of Science, EPSCO, and Cochrane Library from September 2022 to December 2022 using the following query: (chronic subdural hematoma OR cSDH OR subdural hematoma OR subdural hemorrhage OR subdural bleeding) and (drainage OR drain OR drains).
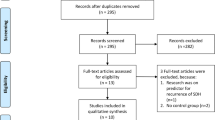
After retrieving citations from electronic databases, we used Endnote to remove duplicates. Then, the retrieved studies were screened in two steps; the first step was to screen titles and abstracts (on the Rayyan database) of all included references independently by two authors at least to assess their relevance to our meta-analysis, then the next step was to screen the full-text of the identities articles for final eligibility to this meta-analysis (Fig. 1). Then, we extracted data from studies accordingly in a uniform sheet for primary and secondary outcomes and for the risk of bias domains (Fig. 2).
Risk of bias assessment is represented in a a traffic light plot and b a summary plot according to the Cochrane risk-of-bias tool, created using robvis [31]
Data extraction
Three authors extracted data from each paper without dependence on another person or thing and collected them in extraction tables. The following data were extracted from each included study: baseline characteristic of the study population (authors, year of publication, mean age, gender, and total number of patients) and summary of the study included (inclusion criteria and exclusion criteria of each study, study arms and main numbers of patients in each arm, age in each arm, and main findings in each study).
Quality assessment
Three authors independently assessed the quality of included studies. We use the risk of bias 2 tool and represent data in an Excel sheet; then, risk of bias assessment is represented in a traffic light plot and summary plot according to the Cochrane risk-of-bias tool, created using robvis (Fig. 2).
Main outcomes
Mortality and recurrence were represented as primary outcomes to know the effect of drainage versus no drainage on the recurrence and mortality rates. Hospital stay, neurological deficits, and GCS scale are secondary outcomes.
Data synthesis
All outcomes were dichotomous and were presented as event and total. The event and total were pooled using Review Manager (version 5.4) in the random model. An outcome with a P value less than 0.05 was considered a significant difference between the two groups. As a diagnostic test accuracy, the estimated overall effect was calculated by MedCalc statistical software.
Assessment of certainty and heterogeneity
Sensitivity analysis was used to conduct a certainty assessment. We excluded one study per time to check the strength of the evidence and ensure the overall results were not altered. We included sensitivity analysis for studies showing different results though sensitivity analysis (Fig. 3). Regarding the heterogeneity, the chi-square test evaluated statistical heterogeneity among studies. Then, the chi-square statistic was used to calculate I-squared. Chi-square with a P value less than 0.1 was considered as significant heterogeneity. Also, the I-square value of more than or equal to 50% indicated high heterogeneity.
Publication bias
To explore the publication bias across studies, we constructed funnel plots to present the relationship between effect size and standard error using Revman version 5.4. Symmetrical plots indicated no publication bias, while asymmetrical plots revealed publication bias (Fig. 4).
Ethical approval
We did not need ethical approval as we collected data from previously ethically approved published studies.
Results
Literature search results and study characteristics
Our search yielded 3844 citations. Of these, 266 full-text articles were retrieved and screened for eligibility after excluding irrelevant studies (n = 3578). Finally, 10 studies (n = 1961 patients) were included in our systematic review [13,14,15,16,17,18,19,20,21,22]. Of these, Carlsen et al. [16] was an observational study and eligible to pool in the meta-analysis (n = 344 patients), and nine [13,14,15, 17,18,19,20,21,22] studies were prospective clinical trial studies (n = 1617 patients; the PRISMA flow diagram in Fig. 1). Additionally, we manually searched references of the included studies, and further studies were eligible for inclusion. A summary of general characteristics of the included articles (e.g., study ID, country, study design, total number of patients, inclusion criteria, exclusion criteria, and main findings of each study) and baseline characteristics (e.g., age and gender of included population) are shown in Table 1.
Risk of bias assessment
Prospective clinical trials assessment by Cochrane tool revealed two studies of overall low risk of bias, one study of some concern, and six studies of high risk of bias. Carlsen et al. [16] was of fair quality risk of bias by Newcastle Ottawa scale (data not shown).
Outcomes
-
A)
Mortality
The overall effect of the analysis of six studies [13,14,15,16, 18, 20] favored drainage over non-drainage in decreasing mortality rate (OR = 0.65, 95% CI 0.43 to 0.97; P = 0.04), with no heterogeneity (I2 = 0%, P = 0.44) (Fig. 3a).
-
B)
Gross focal neurological deficit
No significant differences were observed with regard to gross focal neurological deficit between the two groups (OR = 0.69, 95% CI 0.42 to 1.14; P = 0.14), with mild heterogeneity (I2 = 12%, P = 0.34) (Fig. 3b). The heterogeneity was resolved after excluding Laumer et al. [21] by sensitivity analysis (I2 = 0%, P = 0.66), and a significant association was found between the non-drainage group and gross focal neurological deficit incidence (OR = 0.58, 95% CI 0.37 to 0.89; P = 0.01) [14,15,16, 18, 20, 21] (Fig. 3c).
-
C)
GCS of 15 (at discharge)
No significant differences were observed with regard to GCS of 15 (at discharge) between the two groups (OR = 1.21, 95% CI 0.84 to 1.76; P = 0.30), with no observed heterogeneity (I2 = 2%, P = 0.30) [14, 15, 20, 23] (Fig. 3d).
-
D)
Recurrence
The overall effect of the analysis of nine studies [14,15,16,17,18,19,20,21,22] favored drainage over non-drainage in decreasing recurrence rate (OR = 0.45, 95% CI 0.28 to 0.71; P = 0.0007), with moderate heterogeneity (I2 = 42%, P = 0.09) (Fig. 3e). The heterogeneity was resolved after excluding Gurelik et al. [19] by sensitivity analysis (I2 = 0%, P = 0.50), and results remained significant in favor of drainage (OR = 0.39, 95% CI 0.28 to 0.55; P < 0.00001) (Fig. 3f).
Publication bias assessment
Visual inspections of funnel plots in terms of mortality, gross focal neurological deficit, GCS of 15 at discharge, and recurrence revealed asymmetry. Therefore, there was evidence of potential publication bias (Fig. 4).
Discussion
This systematic review and meta-analysis revealed a significant difference in the outcomes of patients with chronic subdural hematoma who received drainage compared to those who did not. The results indicate that drainage is associated with a lower risk of mortality, a lower risk of recurrence, and a lower incidence of gross focal neurological deficits in patients with chronic subdural hematoma. However, we did not observe any differences in GCS scores between the two groups.
Knowledge on cSDH has markedly expanded in recent decades. The molecular factors contributing to the pathogenesis of cSDH primarily involve inflammatory and angiogenic pathways [24]. Following an initial injury, a sequence of intricate cellular and molecular responses ensues, resulting in the development of a highly vascularized external neomembrane within the subdural space. This neomembrane is characterized by the accumulation of blood, blood degradation products, and extravasation fluid. Immune cell migration toward the site of injury is driven by chemotactic factors such as fibrinogen degradation products, eotaxin, platelet activation factor, and CXCL-8 (interleukin-8 or IL-8). Elevated levels of cytokines including IL-6, hypoxia-inducible factor, tumor necrosis factor alpha, and cyclooxygenase-2 may stimulate the secretion of vascular endothelial growth factor (VEGF), a critical mediator of angiogenesis. Additionally, proteases like matrix metalloproteinase play a role in releasing angiogenic molecules stored in the provisional extracellular matrix. Intracellular signaling pathways, notably the Smad pathway, are activated by transforming growth factor beta, sensitizing cells to external stimulation via growth factors and cytokines. The neomembrane’s high levels of VEGF, placental growth factor, and angiopoietin-2 promote increased vascular permeability through signaling pathways like MEK/ERK and JAK/STAT. This results in ongoing extravasation of plasma proteins, contributing to hematoma volume expansion. The limited presence of anti-inflammatory cytokines, such as IL-10 and IL-13, and factors conducive to establishing a functional vessel network, like platelet-derived growth factor, may contribute to the chronicity of the condition [24].
Our study results were consistent with previous studies that investigated the same effect in terms of recurrence rate, showing that drainage was associated with 55% [10], 49% [25], and 64% [26] lower recurrence rates. Regarding poor functional outcomes, patients treated with drainage had better functional outcomes, Alcalá-Cerra et al. [25] reported similar results, but Peng and Zhu [10] reported insignificant differences. Our study is the first meta-analysis to demonstrate that drainage was associated with 35% fewer mortality rates, an observation previously observed as insignificant [10, 25, 26]. This finding may be due to the increased number of patients and studies included in our study, which almost doubled. We did not analyze complications between drainage and non-drainage, a factor in which all previous studies [10, 25, 26] did not find differences. However, we reported insignificant results between the two groups in GCS scores of 15 at discharge. Table 2 compares our results to those from previously published meta-analysis studies.
It is worth noting that there are many other factors that need to be considered besides the presence or absence of a drain. The optimal orientation for drain placement to minimize pneumocephalus has been investigated in a limited number of studies. For instance, Nakaguchi et al. conducted research and discovered a notable decrease in postoperative pneumocephalus and a reduced recurrence rate in patients in whom the drain was directed anteriorly, specifically toward the frontal region, in comparison to patients with drains placed parietally or occipitally [27]. The rationale behind these findings was attributed to the improved ability to evacuate air collections with a frontal drain orientation, particularly when patients were in a supine position. In another study by Shiomi et al. it was observed that patients with a frontal drain orientation experienced a significantly longer duration before recurrence, and there was a trend toward a lower recurrence rate [28]. Conversely, Ohba et al. conducted their own investigation but did not identify a statistically significant distinction between drain placements in the frontal versus dorsal positions [29]. Additionally, Katsuki et al. noted a correlation between the outer membrane colors (white, red, and yellow) and the histopathological staging from type I to IV, suggesting that the presence of a white outer membrane may pose a risk for recurrence [30]. These findings underscore the significance of comprehending the pathology of cSDH and its connection to endoscopic and surgical observations.
Finally, one of the main strengths of this study is the large sample size, which increases the reliability of the findings. Additionally, the use of meta-analytic techniques allowed us to pool data from multiple studies and synthesize the results statistically rigorously. This helped increase the study’s power and reduce the risk of type I errors. However, some limitations to this study should be noted. First, there is a mild heterogeneity in the included studies, which could limit the generalizability of the findings. This heterogeneity could be due to differences in patient populations, surgical techniques, and postoperative management strategies. Another important consideration is the quality of the studies that were included. Although the inclusion criteria for this study were strict, there was still significant variability in the quality of the included studies. This could have influenced the results and limited the findings’ generalizability.
Conclusion
This systematic review and meta-analysis prove that drainage is a superior treatment for chronic subdural hematoma compared to no drainage. These findings have significant implications for clinical practice and suggest that surgical drainage may benefit patients with chronic subdural hematoma. However, additional high-quality studies are necessary to validate these results and assess the long-term outcomes of this treatment approach in this patient population.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Feghali J, Yang W, Huang J (2020) Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg 141:339–345
Rauhala M, Helén P, Huhtala H, Heikkilä P, Iverson GL, Niskakangas T et al (2020) Chronic subdural hematoma—incidence, complications, and financial impact. Acta Neurochir (Wien) 162:2033–2043
Ducruet AF, Grobelny BT, Zacharia BE, Hickman ZL, DeRosa PL, Anderson K et al (2012) The surgical management of chronic subdural hematoma. Neurosurg Rev 35:155–169
Nakagawa I, Kotsugi M, Yokoyama S, Maeoka R, Tamura K, Takeshima Y et al (2023) Extensive roles and technical advances of middle meningeal artery embolization for chronic subdural hematoma. Neurol Med Chir (Tokyo) 63:327–333
Abdollahifard S, Farrokhi A, Yousefi O, Valibeygi A, Azami P, Mowla A (2022) Particle embolic agents for embolization of middle meningeal artery in the treatment of chronic subdural hematoma: a systematic review and meta-analysis. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci. https://doi.org/10.1177/15910199221125977
Khorasanizadeh M, Shutran M, Garcia A, Enriquez-Marulanda A, Moore JM, Ogilvy CS et al (2022) Middle meningeal artery embolization with isolated use of coils for treatment of chronic subdural hematomas: a case series. World Neurosurg 165:e581–e587
Catapano JS, Ducruet AF, Nguyen CL, Baranoski JF, Cole TS, Majmundar N et al (2021) Middle meningeal artery embolization for chronic subdural hematoma: an institutional technical analysis. J NeuroInterventional Surg 13:657–660
Atefi N, Alcock S, Silvaggio JA, Shankar J (2023) Clinical outcome and recurrence risk of chronic subdural hematoma after surgical drainage. Cureus 15:e35525
Chen C, Xiong Y, Huang X, Guo X, Kang X, Zhou J et al (2023) Subperiosteal/subgaleal drainage vs. subdural drainage for chronic subdural hematoma: a meta-analysis of postoperative outcomes. PLOS ONE 18:e0288872
Peng D, Zhu Y (2016) External drains versus no drains after burr‐hole evacuation for the treatment of chronic subdural haematoma in adults. Cochrane Database of Sys Rev 8:CD011402. https://doi.org/10.1002/14651858.CD011402.pub2. Accessed 11 Sep 2023
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA et al (2020) statement: an updated guideline for reporting systematic reviews. BMJ 2021:372
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds.) (2023) Cochrane handbook for systematic reviews of interventions version 6.4. Cochrane. Available from www.training.cochrane.org/handbook
Guilfoyle MR, Hutchinson PJA, Santarius T (2017) Improved long-term survival with subdural drains following evacuation of chronic subdural haematoma. Acta Neurochir (Wien) 159:903–905
Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, Smielewski P et al (2009) Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet Lond Engl 374:1067–1073
Singh AK, Suryanarayanan B, Choudhary A, Prasad A, Singh S, Gupta LN (2014) A prospective randomized study of use of drain versus no drain after burr-hole evacuation of chronic subdural hematoma. Neurol India 62:169–174
Carlsen JG, Cortnum S, Sørensen JC (2011) Recurrence of chronic subdural haematomata with and without post-operative drainage. Br J Neurosurg 25:388–390
Kutty SA, Johny M (2014) Chronic subdural hematoma: a comparison of recurrence rates following burr-hole craniostomy with and without drains. Turk Neurosurg 24:494–497
Erol FS, Topsakal C, Ozveren MF, Kaplan M, Tiftikci MT (2005) Irrigation vs. closed drainage in the treatment of chronic subdural hematoma. J Clin Neurosci 12:261–3
Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag Ö, Kars HZ (2007) A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci J Can Sci Neurol 34:84–87
Javadi A, Amirjamshidi A, Aran S, Hosseini SH (2011) A randomized controlled trial comparing the outcome of burr-hole irrigation with and without drainage in the treatment of chronic subdural hematoma: a preliminary report. World Neurosurg 75:731–736
Laumer R, Schramm J, Leykauf K (1989) Implantation of a reservoir for recurrent subdural hematoma drainage. Neurosurgery 25:991
Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T (1997) The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg 87:870–875
Wang W, Liu H, Yang J (2017) Burr hole craniostomy irrigation with and without drainage during surgical treatment of chronic subdural hematoma: a retrospective study of 87 cases. Turk Neurosurg 28:748–755
Weigel R, Schilling L, Krauss JK (2022) The pathophysiology of chronic subdural hematoma revisited: emphasis on aging processes as key factor. GeroScience 44:1353–1371
Alcalá-Cerra G, Young AMH, Moscote-Salazar LR, Paternina-Caicedo Á (2014) Efficacy and safety of subdural drains after burr-hole evacuation of chronic subdural hematomas: systematic review and meta-analysis of randomized controlled trials. World Neurosurg 82:1148–1157
Liu W, Bakker NA, Groen RJM (2014) Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg 121:665–673
Nakaguchi H, Tanishima T, Yoshimasu N (2000) Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg 93:791–795
Shiomi N, Hashimoto N, Tsujino H, Takahashi Y, Murakami M (2002) Mineura K [Relationship of direction of drainage tube and recurrence in chronic subdural hematoma]. No Shinkei Geka 30:823–827
Ohba S, Kinoshita Y, Nakagawa T, Murakami H (2013) The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev 36:145–9; discussion 149–150
Katsuki M, Kakizawa Y, Wada N, Yamamoto Y, Uchiyama T, Nakamura T et al (2020) Endoscopically observed outer membrane color of chronic subdural hematoma and histopathological staging: white as a risk factor for recurrence. Neurol Med Chir (Tokyo) 60:126–135
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12:55–61
Acknowledgements
Mohamed Abouzid is a participant of STER Internationalization of Doctoral Schools Program from NAWA Polish National Agency for Academic Exchange No. PPI/STE/2020/1/00014/DEC/02.
Author information
Authors and Affiliations
Contributions
AA and MaA: conceptualization and methodology. AMS, BJ, IS, and NMA-d: investigation and data curation. NMA-d: formal analysis. AMS, BJ, IS, NMA-d, and MA: writing—original draft. AA and MA: supervision. MaA: project administration. MA: writing—review and editing. All authors read and approved the final content.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aljabali, A., Sharkawy, A.M., Jaradat, B. et al. Drainage versus no drainage after burr-hole evacuation of chronic subdural hematoma: a systematic review and meta-analysis of 1961 patients. Neurosurg Rev 46, 251 (2023). https://doi.org/10.1007/s10143-023-02153-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02153-7