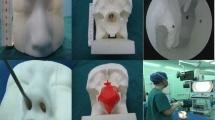
Abstract
Endoscopic transsphenoidal surgery is a novel surgical technique requiring specific training. Different models and simulators have been recently suggested for it, but no systematic review is available. To provide a systematic and critical literature review and up-to-date description of the training models or simulators dedicated to endoscopic transsphenoidal surgery. A search was performed on PubMed and Scopus databases for articles published until February 2023; Google was also searched to document commercially available. For each model, the following features were recorded: training performed, tumor/arachnoid reproduction, assessment and validation, and cost. Of the 1199 retrieved articles, 101 were included in the final analysis. The described models can be subdivided into 5 major categories: (1) enhanced cadaveric heads; (2) animal models; (3) training artificial solutions, with increasing complexity (from “box-trainers” to multi-material, ct-based models); (4) training simulators, based on virtual or augmented reality; (5) Pre-operative planning models and simulators. Each available training model has specific advantages and limitations. Costs are high for cadaver-based solutions and vary significantly for the other solutions. Cheaper solutions seem useful only for the first stages of training. Most models do not provide a simulation of the sellar tumor, and a realistic simulation of the suprasellar arachnoid. Most artificial models do not provide a realistic and cost-efficient simulation of the most delicate and relatively common phase of surgery, i.e., tumor removal with arachnoid preservation; current research should optimize this to train future neurosurgical generations efficiently and safely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic transsphenoidal surgery is a novel surgical technique that recently evolved in endoscopic skull base surgery [1]. As we have learned to exploit the advantages of the relatively large median and paramedian corridors to the skull base [2,3,4,5], the indications for this surgery have been expanding, together with its complexity.
It is well recognized that endoscopic transsphenoidal surgery has a long learning curve [6], which requires integrated and specific training [7]. Though traditional neurosurgical training is still primarily based on experience in the operating room, many complementary methods are now available. The cadaver laboratory has been classically used to acquire basic technical skills and knowledge of detailed surgical anatomy. Still, high maintenance costs and the challenge of simulating pathologies might limit its utility. Thanks to 3D printing technologies, it has become possible to create customized models replicating normal and pathological anatomy [8]. Furthermore, thanks to virtual reality (VR) development, simulators may provide a repeatable experience in a more complex anatomical environment. In addition, the development of augmented reality (AR) simulators might enhance the quality of training.
In this evolving scenario, this review aims to provide a systematic and up-to-date description of the training solutions for endoscopic transsphenoidal surgery, along with their technical details, costs, utility for surgical skills development, and validation.
Material and methods
Search strategy
A systematic review, following the PRISMA 2020 statement [9, 10], was performed by searching articles published until February 2023 on PubMed and Scopus, with the following keywords: training AND (transsphenoidal OR transnasal) AND (phantom OR simulator OR model); physical AND (simulator OR phantom OR model) AND (endoscopic endonasal); (Pituitary OR hypophysis) AND surgery AND training AND (model OR phantom OR simulator); (transsphenoidal) AND ((3D print*) OR (three dimension* print*)); ((3D print*) OR (three dimension* print*)) AND tumor AND pituitary OR hypophysis; (Endoscopic endonasal) AND ((3D print*) OR (three dimension* print*)); (neurosurgical) AND training AND ((phantom) OR (model) OR (simulator)) AND (3D print*) OR (three dimension* print*); (Skull base) AND (surgery) AND (training) AND ((model) OR (phantom) OR (simulator)).
Additional references and models or simulators used for training in endoscopic transsphenoidal surgery were identified by reference analysis and investigations on the web using the Google search engine.
Inclusion and exclusion criteria
Inclusion criteria were as follows: English Language, training models, or simulators for endoscopic transsphenoidal surgery.
Exclusion criteria were the following: non-English language, papers unavailable at our libraries, models/simulators for other surgical interventions, and other studies (e.g., reviews with no novel data).
Quality assessment and data extraction
Articles were imported into the reference management software Zotero (version 6.0.8), and duplicates were removed. AM and GS examined the title and abstract of the retrieved records, and non-relevant citations were excluded. Any disagreement was resolved by discussion between the reviewers. For each selected study, an accurate full-text analysis was performed to extract the following information about the training model or simulator, when available: reproduced anatomy, data on training and validation studies, and costs.
The selected studies were divided into the following categories (Fig. 1):
-
1.
Enhanced cadaver models (ECH);
-
2.
Animal models (AM);
-
3.
Training models;
-
3.1
Box-Trainers;
-
3.2
CT-based: mono-material model (m), multi-material model (M), and the “EggHead”;
-
3.1
-
4.
Training Simulators: virtual reality (VR) simulator and augmented reality (AR) simulator;
-
5.
Preoperative planning models/simulators.
The difference between “model” and “simulator” is that simulators are models in a virtual reality environment and with real-time feedback for the surgeon.
Each training model/simulator was listed in a table based on the category. In addition, each model was described in the table reporting the following data when available:
-
1.
First author and year of publication for academic reports, or name of the developers and nation, for commercially available models/simulator (CA);
-
2.
whether the model included the tumor (T) and the arachnoid membrane (A) in their model;
-
3.
Simulated tasks for which the model was conceived and used;
-
4.
Assessment or validation of the model;
-
5.
The reported cost of the used materials or the retail price.
For the CT-based training models, a 5-point sub-column was added to evaluate their anatomical reliability and defined “anatomy score.” The sub-column score gives an overall evaluation of the anatomical accuracy of the model; points are given according to the design of the model: +1 point per mono-material (m) models or +2 points for multi-material (M) ones M; +1 or +2 points according to the degree of reproduced details, such as the skin, dura mater, optic nerve, or ICA; and +1 point if the tumor or the arachnoid are reproduced.
Results
The initial literature search yielded 1199 articles: 675 from PubMed and 524 from Scopus. Of these articles, 568 were removed before screening because they were duplicates. The remaining 631 articles were screened and evaluated by title. At this point, 380 articles were excluded, and a full-text screening was performed to determine if the remaining 251 articles met the inclusion criteria. Of the 251 articles identified for retrieval, 2 were removed (because the full text was not available). A total of 249 reports were screened for eligibility and 181 were removed because they did not meet the inclusion criteria, specifically 15 were removed because of language; 8 were removed because of experience with a pre-existing model or simulator; 76 were removed because the model/simulator described was used to simulate other surgeries; 51 were removed because no model/simulator was described in the reports; 9 were removed because they were designed for planning; and 22 were removed because they were previous reviews of the literature. Finally, 101 reports were included in this systematic review, including 6 articles retrieved from previous papers [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] and 28 websites (Fig. 2).
Enhanced cadaver models
The cadaver-based training models can be divided into three main groups, according to the training experience they provide (Table 1): tumor resection [33, 34, 38, 46], management of ICA injury [35,36,37, 41, 43, 44], and CSF leak repair [41, 42, 45, 47].
The tumor resection models are designed to train neurosurgeons to resect a sellar tumor [33, 34, 38]. The idea is based on the work of Gragnaniello et al. who injected resin into the sella turcica to mimic the texture and location of a pituitary tumor [33].
In ICA injury models, a red-dyed solution is pumped into the arterial system to mimic blood [35,36,37, 41, 43, 44]; an ICA lesion is caused, and the surgeon can be trained to deal with it.
The CSF models are obtained by perfusing a water-based solution in the subarachnoid or subdural space so that the surgeon can be trained in skull base reconstruction [39, 40, 42, 45].
Although anatomical specimens are intuitively used at their best only once, some models can be used multiple times for training, lowering their total cost [37, 41, 48]. For example, Mladina et al. [49] reported a cost of $1520 per resident.
Animal models
These models use animals to provide training, mainly on surgical instrumentation handling (Table 2). The animals include Wistar rats [50], lambs [49, 54, 56, 58], and sheep [55, 57] and also one hybrid model specifically designed to manage ICA rupture with a live sheep [51,52,53].
Artificial models
By definition, these models are fabricated artificially. They can be divided into two major categories: the first is represented by the so-called box-trainers (Table 3), while the second comprises anatomically more realistic models (Table 4). Artificial training models are considered the most cost-effective alternative to cadaver-based training [89]. Tables 3 and 4 report each model costs when available.
Box-trainer
If compared to CT-based models, the anatomical accuracy of box-trainers is significantly lower, but they are generally easier to fabricate and cheaper.
The models under this category are characterized by a box with two holes representing the head and the nostrils. Different materials and training modules can be used inside the box, e.g., chicken wing or tangerine [60, 62, 66, 68], rings, and pegs, to create specifically designed exercises [59, 61, 63,64,65, 69]. These models aim to develop the surgeon’s dexterity [68]. The box-trainers are reported in Table 3.
CT-based models
These training models are developed from patient-specific CT data (Table 4). The overall level of anatomical accuracy is strongly related to the design, materials, and technology used.
Some are mono-material solutions [70, 72, 81], while others are multi-material to reproduce the different tissues of the human head more accurately [66, 71, 73, 74, 76,77,78,79,80, 82, 83, 85,86,87,88, 101, 102, 105].
A brilliant and cheap solution frequently incorporated in CT-based models is the “EggHead,” described by Engel et al. [77]: a chicken or quail egg reproduces the sellar region anatomy [46, 67, 72, 74, 77, 80, 85]. The eggshell mimics the sphenoid bone, the vitelline membrane is the dura mater, and the albumen and yolk represent the contents of the sella. According to Wen et al. [80], the egg may be raw or soft-boiled.
Among multi-material training models, some are commercially available, such as SIMONT by ProDelphus [91], Kezlex: A22 [98], A39 [99], and A43 [100] by Japan Medical Company [96]. The Sinus Model Otorhino Neuro Trainer, SIMONT - Otorhino Surgical Trainer, is the training model developed which allows the performing of many neurosurgical operations [93], including removing the pituitary adenoma. One of the most innovative features is Neoderma®, the material developed by Pro Delphus used to mimic the mechanical properties of the skin and the mucous membranes [94, 95, 104]. The model is available on the website [92] for US$ 3798.00, while the portable version costs US$ 1630. In the literature, its use has been described by Valentine et al. [51,52,53].
Kezlex is a series of training models developed by Japan Medical Company [96]. Among all the training solutions [97], the most pertinent are models A22 [98], A39 [99], and A43 [100]. Oyama et al. described their experience with the A22 for various neurosurgical approaches. Maza et al. [101] described the A43 model. This training model was developed to help the neurosurgeon deal with a catastrophic ICA injury. The cost is not reported on the website, but Muto et al. [102] reported in their article the cost of the A43 model of $4000 plus $250 for the reusable platform.
Simulators
Training simulators can be divided into two categories, as they are either based on virtual (VR) or augmented reality (AR). Table 5 reports the relative costs of each model when available.
Virtual reality
VR simulators consist of a PC with a virtual environment software that represents the patient’s data, and the surgeons interact with it by simulating actual surgeries using joysticks [106, 107, 109], special haptic devices [108, 115, 118, 121, 147], or surgical instruments [114]. Virtual reality simulators are a technologically advanced alternative to train surgeons to perform complex surgeries before they enter the operating room [3, 60].
Two different VR simulators were found to be available online, the NeuroVR and Voxel-Man Sinus.
The NeuroTouch-Endo, now NeuroVR, is the training simulator developed by NRC and NeuroSim [116] (Canada); it is a VR simulator that simulates endoscopic transnasal procedures [132] with MRI data for patient-specific features. In addition, it has haptic devices that provide force feedback [115, 133], and it is available on the CAE website [134].
The Voxel-Man Sinus [126] is the training simulator developed by the University Medical Center Hamburg-Eppendorf (Germany) [127] for paranasal sinus surgery [128]. The Voxel-Man provides an accurate haptic and visual representation of surgery and is based on standard PC hardware [129]. The Voxel-Man can be purchased [130] for $ 145,255.95$ [131].
Augmented reality simulators
AR simulators are VR simulators where surgeons interact with a physical, CT-based [75, 117, 120, 122, 123], or cadaver [110,111,112,113,114, 117] head. Additionally, Cai et al. [125] developed an application that can be used for AR simulators. Two models were available for purchase, the Phacon Sinus Trainer and the TNS Box.
The PHACON Sinus Trainer comprises a series of simulators developed by Phacon GmbH (Germany) [135]. The most suitable for this review were found with the web research: the [S-00005] PHACON Sinus Trainer [137], available at 8.910€ [140], and the [S-00007] PHACON Sinus Assistant [136], purchasable at 1.870€ [139]. The module for the transnasal approach, the [SN-ah] PHACON Sinus Patient “Meyer” – pituitary tumor, can be purchased separately for 290€ [141]. The simulator consists of a multi-material modular head connected via visual registration to specially developed software that assists the neurosurgeon by providing CT data displayed as a virtual 3D model; it can automatically detect injuries to high-risk structures.
The TNS Box is one of the multiple simulators developed by UpSurgeOn [142]. It consists of an anatomically accurate modular and multi-material simulator designed explicitly for the transsphenoidal approach to the pituitary gland. The simulator comprises an external box with a disposable nasal cavity and a face mask on the front. The TNS is provided with an App available on the App Store or Google Play, which improves the training experience with a virtual reality environment [143]. The TNS is now available at UpSurgeOn website [145] at €599–€699. It is also possible to purchase disposable nasal cavities separately [146]. Two articles reported a positive experience with the simulators [144, 148].
Models and simulators for surgical planning
Table 6 reports models and simulators conceived for surgical planning, which are not included in this systematic review but might help neurosurgeons improve their knowledge on the subject [28, 149,150,151,152,153,154, 156,157,158].
Discussion
It is recognized that the endoscopic endonasal transsphenoidal approach has a long learning curve [6]. To ensure safe and effective surgery, it is crucial to have excellent hand-eye coordination under the endoscopic vision and make sound clinical and intraoperative judgments. The required confidence can only be achieved after many surgeries in the operating room. However, this learning process can be sped up with proper training in a safe environment outside the operating room.
This systematic review aimed to show all alternatives for training in endoscopic transsphenoidal surgery. We found four categories of training systems: enhanced cadaver head training models, animal models, training models (CT-based, box-trainer, and EggHead), and training simulators (virtual reality and augmented reality).
Human cadaver heads remain the gold standard for training: the anatomical reliability is still higher if compared to every other option [21]. However, their low availability [18, 40, 45] and the fact that they are suitable for limited training experience make them an expensive and not easily accessible option [42]: the cost of one human cadaveric head ranges from almost 600$ [48] to 1000$ [79], while Mladina et al. [49] reported a cost of 1520$ per resident for training. In addition, the maintenance costs of anatomy laboratories are high [17]. Using animal heads is a cheap and readily available option, but the anatomy is divergent [56]. Nevertheless, they can be considered a good alternative as an inexpensive and simple system to teach residents the dexterity required to fully exploit the more expensive cadaver head, as stated by Mladina et al. [49]. Their main advantages are the costs which are lower than 6$ [49, 55, 56], making them the cheapest solution for initial training.
Compared to human and animal specimens, training models have the advantage of being versatile. The developers can choose the anatomical accuracy level they want to obtain, which is directly related to the costs of the system. Modular solutions, in which not all parts are disposable, are a way to optimize the costs of this solution.
Among the different solutions, the box-trainers are cheaper and easier to fabricate. However, the low degree of anatomical accuracy makes them suitable as a first tool to teach how to handle surgical instruments in the narrow space of the nasal cavities, and they can be a useful first experience before training with more expensive models like the cadaveric head [68].
CT-based training models, on the other hand, potentially have a significantly higher level of accuracy related to the design complexity and the background knowledge required. The EggHead represents a brilliant solution as it mimics the sellar region with a chicken or quail egg in an economical and repeatable way [46, 72, 74, 77, 80, 85]. What needs to be added is a reproduction of blood and CSF [76]; the latter was implemented only in the training model of Mashala et al. [89]. Costs are generally low but cannot be compared to each other due to the different criteria by which they were determined by the authors, as reported in Table 4. They can be divided into three categories: cost per model, material cost, and production cost.
VR training simulators provide visually the most complete experience to neurosurgeons. Their main advantage is the fact that the simulation can ideally be repeated an infinite number of times [17]. In addition, some of them also have a real-time feedback system that provides information about the position of the instruments, the level of forces reached, and the performances of the trainees [18, 115, 129, 133]. However, the lack of a “physical head” where to perform the surgery can be limiting, even if many sensors and haptic devices have been studied and added [121]. Another defect of some VR systems is the low quality of the visual effects and the fact that the instruments used during training sessions differ from those used in the operating room [106,107,108,109, 116, 118, 121, 124, 131]. The initial costs of VR training simulators are the highest among the different solutions; i.e., the Voxel-Man Sinus training simulator is available for 145,255.95$ [130, 131]. However, the fact that surgeries can be simulated an indefinite number of times makes the cost of a single training session low if the system is used frequently.
AR simulators with cadaver heads may be the best solution for residents as they provide the best anatomy from the cadaver head and real-time feedback from the VR environment [110,111,112, 117, 156]. However, they may also be the most expensive solutions: a better trade-off to reduce cost may be an AR simulator based on a multi-material head. The costs available for the training simulators are those of the Phacon, 8910€ [140], or 1870€ [139] and 290€ [141] for the cartridge, and those of the TNS, 599–699€ [145], plus the costs of the disposable cartridge, which it is not reported. Similar to CT-based training models, developing VR and AR simulators requires a high level of knowledge.
Finally, this review documents what is missing in most training solutions. Most are dedicated to the phase of the approach in surgery, while only a minority have developed simulators for sellar tumors and suprasellar arachnoid. Except for VR simulators, where the pituitary adenoma was implemented virtually, the sellar tumor has been simulated only in a few models using different materials. In addition, ECH models have been modified to allow training for dealing with ICA intra-operative rupture and CSF leak. We believe it might be of interest to develop a modular training model that provides a realistic simulation of both sellar tumors and suprasellar arachnoid to provide a cost-efficient way to train future generations not only in the surgical approach but also in the management of sellar tumors of different consistencies and the preservation of the arachnoid.
Limits of the study
The limit of this systematic review could be the lack of some data of the training models/simulators (e.g., the cost of the training model) and therefore the difficulty of comparing the models. Furthermore, not all models that are being developed are available at the moment. We expect that further improvements will be made soon in the field.
Conclusions
The training solutions for endoscopic transsphenoidal surgery are cadaveric (human or animal) or artificial models and virtual reality simulators. Human cadaveric specimens constitute the gold standard, as they provide a realistic environment, which specific modifications for managing ICA rupture, CSF leak, and tumor removal can enhance. Their availability is though relatively low due to relatively high costs. Virtual reality simulators and artificial models provided an excellent alternative. However, the lack of haptic realism and anatomical fidelity makes them ideal for learning the basics. Augment reality applied to cadaver-based models is an exciting solution that might be further developed in the near future.
Most artificial models do not provide a realistic and cost-efficient simulation of the most delicate and relatively common phase of surgery, i.e., tumor removal with arachnoid preservation; current research should optimize this to train future neurosurgical generations efficiently and safely.
Data availability
This review was performed by searching articles on Pubmed and Scopus.
References
Doglietto F, Prevedello DM, Jane JA, Han J, Laws ER (2005) Brief history of endoscopic transsphenoidal surgery--from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus 19:E3
Agosti E, Saraceno G, Qiu J, Buffoli B, Ferrari M, Raffetti E, Belotti F, Ravanelli M, Mattavelli D, Schreiber A, Hirtler L, Rodella LF, Maroldi R, Nicolai P, Gentili F, Kucharczyk W, Fontanella MM, Doglietto F (2020) Quantitative anatomical comparison of transnasal and transcranial approaches to the clivus. Acta Neurochir (Wien) 162:649–660. https://doi.org/10.1007/s00701-019-04152-4
Belotti F, Doglietto F, Schreiber A, Ravanelli M, Ferrari M, Lancini D, Rampinelli V, Hirtler L, Buffoli B, Bolzoni Villaret A, Maroldi R, Rodella LF, Nicolai P, Fontanella MM (2018) Modular classification of endoscopic endonasal transsphenoidal approaches to sellar region: anatomic quantitative study. World Neurosurg 109:e281–e291. https://doi.org/10.1016/j.wneu.2017.09.165
Doglietto F, Ferrari M, Mattavelli D, Belotti F, Rampinelli V, Kheshaifati H, Lancini D, Schreiber A, Sorrentino T, Ravanelli M, Buffoli B, Hirtler L, Maroldi R, Nicolai P, Rodella LF, Fontanella MM (2018) Transnasal endoscopic and lateral approaches to the clivus: a quantitative anatomic study. World Neurosurg 113:e659–e671. https://doi.org/10.1016/j.wneu.2018.02.118
Schreiber A, Ferrari M, Rampinelli V, Doglietto F, Belotti F, Lancini D, Ravanelli M, Rodella LF, Fontanella MM, Nicolai P (2017) Modular endoscopic medial maxillectomies: quantitative analysis of surgical exposure in a preclinical setting. World Neurosurg 100:44–55
Kenan K, İhsan A, Dilek O, Burak C, Gurkan K, Savas C (2006) The learning curve in endoscopic pituitary surgery and our experience. Neurosurg Rev 29:298–305. https://doi.org/10.1007/s10143-006-0033-9
Choudhury N, Gélinas-Phaneuf N, Delorme S, Del Maestro R (2013) Fundamentals of neurosurgery: virtual reality tasks for training and evaluation of technical skills. World Neurosurg 80:e9–e19. https://doi.org/10.1016/j.wneu.2012.08.022
Hsieh T-Y, Cervenka B, Dedhia R, Strong EB, Steele T (2018) Assessment of a patient-specific, 3-dimensionally printed endoscopic sinus and skull base surgical model. JAMA Otolaryngol - Head Neck Surg 144:574–579. https://doi.org/10.1001/jamaoto.2018.0473
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160. https://doi.org/10.1136/bmj.n160
Baby B, Singh R, Singh R, Suri A, Arora C, Kumar S, Kalra PK, Banerjee S (2020) A review of physical simulators for neuroendoscopy skills training. World Neurosurg 137:398–407. https://doi.org/10.1016/j.wneu.2020.01.183
Baby B, Singh R, Suri A, Dhanakshirur RR, Chakraborty A, Kumar S, Kalra PK, Banerjee S (2020) A review of virtual reality simulators for neuroendoscopy. Neurosurg Rev 43:1255–1272. https://doi.org/10.1007/s10143-019-01164-7
Bajaj J, Chandra PS (2020) Recent developments in endoscopic endonasal approach for pituitary adenomas. Neurol India 68:S79–S84. https://doi.org/10.4103/0028-3886.287671
Blohm JE, Salinas PA, Avila MJ, Barber SR, Weinand ME, Dumont TM (2022) Three-dimensional printing in neurosurgery residency training: a systematic review of the literature. World Neurosurg 161:111–122. https://doi.org/10.1016/j.wneu.2021.10.069
Chan S, Conti F, Salisbury K, Blevins NH (2013) Virtual reality simulation in neurosurgery: technologies and evolution. Neurosurgery 72:A154–A164. https://doi.org/10.1227/NEU.0b013e3182750d26
Cohen AR, Lohani S, Manjila S, Natsupakpong S, Brown N, Cavusoglu MC (2013) Virtual reality simulation: basic concepts and use in endoscopic neurosurgery training. Childs Nerv Syst 29:1235–1244. https://doi.org/10.1007/s00381-013-2139-z
James J, Irace AL, Gudis DA, Overdevest JB (2022) Simulation training in endoscopic skull base surgery: a scoping review. World J Otorhinolaryngol - Head Neck Surg 8:73–81. https://doi.org/10.1002/wjo2.11
Kim DH, Kim Y, Park J-S, Kim SW (2019) Virtual reality simulators for endoscopic sinus and skull base surgery: the present and future. Clin Exp Otorhinolaryngol 12:12–17. https://doi.org/10.21053/ceo.2018.00906
Kin T, Nakatomi H, Shono N, Nomura S, Saito T, Oyama H, Saito N (2017) Neurosurgical virtual reality simulation for brain tumor using high-definition computer graphics: a review of the literature. Neurol Med Chir (Tokyo) 57:513–520. https://doi.org/10.2176/nmc.ra.2016-0320
Langridge B, Momin S, Coumbe B, Woin E, Griffin M, Butler P (2018) Systematic review of the use of 3-dimensional printing in surgical teaching and assessment. J Surg Educ 75:209–221. https://doi.org/10.1016/j.jsurg.2017.06.033
Lavigne P, Yang N (2020) Training and surgical simulation in skull base surgery: a systematic review. Curr Otorhinolaryngol Rep 8:154–159. https://doi.org/10.1007/s40136-020-00280-z
Li H, Lu L, Li N, Zi L, Wen Q (2022) Application of three-dimensional (3D) printing in neurosurgery. Adv Mater Sci Eng 2022. https://doi.org/10.1155/2022/8015625
Low CM, Morris JM, Price DL, Matsumoto JS, Stokken JK, O’Brien EK, Choby G (2019) Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy 33:770–781. https://doi.org/10.1177/1945892419866319
Maclachlan LR, Alexander H, Forrestal D, Novak JI, Redmond M (2021) Properties and characteristics of three-dimensional printed head models used in simulation of neurosurgical procedures: a scoping review. World Neurosurg 156:133–146.e6. https://doi.org/10.1016/j.wneu.2021.09.079
Neubauer A, Wolfsberger S (2013) Virtual endoscopy in neurosurgery: a review. Neurosurgery 72:A97–A106. https://doi.org/10.1227/NEU.0b013e31827393c9
Patel EA, Aydin A, Cearns M, Dasgupta P, Ahmed K (2020) A Systematic Review of Simulation-Based Training in Neurosurgery, Part 1: Cranial Neurosurgery. World Neurosurg 133:e850–e873. https://doi.org/10.1016/j.wneu.2019.08.262
Randazzo M, Pisapia JM, Singh N, Thawani JP (2016) 3D printing in neurosurgery: a systematic review. Surg Neurol Int 7:S801–S809. https://doi.org/10.4103/2152-7806.194059
Shinomiya A, Shindo A, Kawanishi M, Miyake K, Nakamura T, Matsubara S, Tamiya T (2018) Usefulness of the 3D virtual visualization surgical planning simulation and 3D model for endoscopic endonasal transsphenoidal surgery of pituitary adenoma: technical report and review of literature. Interdiscip Neurosurg Adv Tech Case Manag 13:13–19. https://doi.org/10.1016/j.inat.2018.02.002
Thavarajasingam SG, Vardanyan R, Arjomandi Rad A, Thavarajasingam A, Khachikyan A, Mendoza N, Nair R, Vajkoczy P (2022) The use of augmented reality in transsphenoidal surgery: a systematic review. Br J Neurosurg 36:457–471. https://doi.org/10.1080/02688697.2022.2057435
Thiong’o GM, Bernstein M, Drake JM (2021) 3D printing in neurosurgery education: a review. 3D Print Med 7:9. https://doi.org/10.1186/s41205-021-00099-4
Tomlinson SB, Hendricks BK, Cohen-Gadol A (2019) Immersive three-dimensional modeling and virtual reality for enhanced visualization of operative neurosurgical anatomy. World Neurosurg 131:313–320. https://doi.org/10.1016/j.wneu.2019.06.081
Vakharia VN, Vakharia NN, Hill CS (2016) Review of 3-dimensional printing on cranial neurosurgery simulation training. World Neurosurg 88:188–198. https://doi.org/10.1016/j.wneu.2015.12.031
Gragnaniello C, Nader R, van Doormaal T, Kamel M, Voormolen EHJ, Lasio G, Aboud E, Regli L, Tulleken CAF, Al-Mefty O (2010) Skull base tumor model. J Neurosurg 113:1106–1111. https://doi.org/10.3171/2010.3.JNS09513
Berhouma M, Baidya NB, Ismaïl AA, Zhang J, Ammirati M (2013) Shortening the learning curve in endoscopic endonasal skull base surgery: a reproducible polymer tumor model for the trans-sphenoidal trans-tubercular approach to retro-infundibular tumors. Clin Neurol Neurosurg 115:1635–1641. https://doi.org/10.1016/j.clineuro.2013.02.013
Pham M, Kale A, Marquez Y, Winer J, Lee B, Harris B, Minnetti M, Carey J, Giannotta S, Zada G (2014) A perfusion-based human cadaveric model for management of carotid artery injury during endoscopic endonasal skull base surgery. J Neurol Surg Part B Skull Base 75:309–313. https://doi.org/10.1055/s-0034-1372470
Ciporen JN, Lucke-Wold B, Mendez G, Cameron WE, McCartney S (2017) Endoscopic management of cavernous carotid surgical complications: evaluation of a simulated perfusion model. World Neurosurg 98:388–396. https://doi.org/10.1016/j.wneu.2016.11.018
Pacca P, Jhawar SS, Seclen DV, Wang E, Snyderman C, Gardner PA, Aboud E, Fernandez-Miranda JC (2017) “Live cadaver” model for internal carotid artery injury simulation in endoscopic endonasal skull base surgery. Oper Neurosurg Hagerstown Md 13:732–738. https://doi.org/10.1093/ons/opx035
Gagliardi F, Chau AM, Mortini P, Caputy AJ, Gragnaniello C (2018) Skull base neuroendoscopic training model using a fibrous injectable tumor polymer and the nico myriad. J Craniofac Surg 29:e25–e28. https://doi.org/10.1097/SCS.0000000000004042
AlQahtani A, Albathi A, Castelnuovo P, Alfawwaz F (2021) Cerebrospinal fluid leak repair simulation model: face, content, and construct validation. Am J Rhinol Allergy 35:264–271. https://doi.org/10.1177/1945892420952262
AlQahtani AA, Albathi AA, Alhammad OM, Alrabie AS (2018) Innovative real CSF leak simulation model for rhinology training: human cadaveric design. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg 275:937–941. https://doi.org/10.1007/s00405-018-4902-y
Shen J, Hur K, Zhang Z, Minneti M, Pham M, Wrobel B, Zada G (2018) Objective validation of perfusion-based human cadaveric simulation training model for management of internal carotid artery injury in endoscopic endonasal sinus and skull base surgery. Oper Neurosurg Hagerstown Md 15:231–238. https://doi.org/10.1093/ons/opx262
Christian EA, Bakhsheshian J, Strickland BA, Fredrickson VL, Buchanan IA, Pham MH, Cervantes A, Minneti M, Wrobel BB, Giannotta S, Zada G (2018) Perfusion-based human cadaveric specimen as a simulation training model in repairing cerebrospinal fluid leaks during endoscopic endonasal skull base surgery. J Neurosurg 129:792–796. https://doi.org/10.3171/2017.5.JNS162982
Donoho DA, Johnson CE, Hur KT, Buchanan IA, Fredrickson VL, Minneti M, Zada G, Wrobel BB (2019) Costs and training results of an objectively validated cadaveric perfusion-based internal carotid artery injury simulation during endoscopic skull base surgery. Int Forum Allergy Rhinol 9:787–794. https://doi.org/10.1002/alr.22319
Donoho DA, Pangal DJ, Kugener G, Rutkowski M, Micko A, Shahrestani S, Brunswick A, Minneti M, Wrobel BB, Zada G (2021) Improved surgeon performance following cadaveric simulation of internal carotid artery injury during endoscopic endonasal surgery: training outcomes of a nationwide prospective educational intervention. J Neurosurg 1–9. https://doi.org/10.3171/2020.9.JNS202672
Mattavelli D, Ferrari M, Rampinelli V, Schreiber A, Buffoli B, Deganello A, Rodella LF, Fontanella MM, Nicolai P, Doglietto F (2020) Development and validation of a preclinical model for training and assessment of cerebrospinal fluid leak repair in endoscopic skull base surgery. Int Forum Allergy Rhinol 10:89–96. https://doi.org/10.1002/alr.22451
Li C, Zhu H, Zheng J, Liu C, Gui S, Zhao P, Bai J, Zhang Y (2022) Development and application of three steps training mode for endoscopic transnasal transsphenoidal approach. J Craniofac Surg 33:1554–1558. https://doi.org/10.1097/SCS.0000000000008257
AlQahtani A, London NR, Castelnuovo P, Locatelli D, Stamm A, Cohen-Gadol AA, Elbosraty H, Casiano R, Morcos J, Pasquini E, Frank G, Mazzatenta D, Barkhoudarian G, Griffiths C, Kelly D, Georgalas C, Janakiram N, Nicolai P, Prevedello DM, Carrau RL (2020) Assessment of factors associated with internal carotid injury in expanded endoscopic endonasal skull base surgery. JAMA Otolaryngol Head Neck Surg 146:364–372. https://doi.org/10.1001/jamaoto.2019.4864
Ciporen J, Lucke-Wold B, Dogan A, Cetas JS, Cameron WE (2016) Dual endoscopic endonasal transsphenoidal and precaruncular transorbital approaches for clipping of the cavernous carotid artery: a cadaveric simulation. J Neurol Surg Part B Skull Base 77:485–490. https://doi.org/10.1055/s-0036-1584094
Mladina R, Skitarelić N, Cingi C, Chen L, Muluk NB (2018) The validity of training endoscopic sinus and skull base surgery techniques on the experimental head model. J Craniofac Surg 29:498–501. https://doi.org/10.1097/SCS.0000000000004208
Fernandez-Miranda JC, Barges-Coll J, Prevedello DM, Engh J, Snyderman C, Carrau R, Gardner PA, Kassam AB (2010) Animal model for endoscopic neurosurgical training: technical note. Minim Invasive Neurosurg MIN 53:286–289. https://doi.org/10.1055/s-0030-1269927
Valentine R, Padhye V, Wormald P-J (2016) Simulation training for vascular emergencies in endoscopic sinus and skull base surgery. Otolaryngol Clin North Am 49:877–887. https://doi.org/10.1016/j.otc.2016.02.013
Valentine R, Padhye V, Wormald P-J (2016) Management of arterial injury during endoscopic sinus and skull base surgery. Curr Opin Otolaryngol Head Neck Surg 24:170–174. https://doi.org/10.1097/MOO.0000000000000239
Valentine R, Wormald P-J (2011) A vascular catastrophe during endonasal surgery: an endoscopic sheep model. Skull Base 21:109–114. https://doi.org/10.1055/s-0031-1275255
Mladina R, Castelnuovo P, Locatelli D, Vuković KĐ, Skitarelić N (2013) Training cerebrospinal fluid leak repair with nasoseptal flap on the lamb’s head. ORL 75:32–36. https://doi.org/10.1159/000347080
Awad Z, Touska P, Arora A, Ziprin P, Darzi A, Tolley NS (2014) Face and content validity of sheep heads in endoscopic rhinology training. Int Forum Allergy Rhinol 4:851–858. https://doi.org/10.1002/alr.21362
Skitarelić N, Mladina R (2015) Lamb’s head: the model for novice education in endoscopic sinus surgery. World J Methodol 5:144–148. https://doi.org/10.5662/wjm.v5.i3.144
Isaacson G, Ianacone DC, Wolfson MR (2015) Ex vivo ovine model for pediatric flexible endoscopy training. Int J Pediatr Otorhinolaryngol 79:2196–2199. https://doi.org/10.1016/j.ijporl.2015.10.002
Mallmann LB, Piltcher OB, Isolan GR (2016) The lamb’s head as a model for surgical skills development in endonasal surgery. J Neurol Surg Part B Skull Base 77:466–472. https://doi.org/10.1055/s-0036-1583186
Hirayama R, Fujimoto Y, Umegaki M, Kagawa N, Kinoshita M, Hashimoto N, Yoshimine T (2013) Training to acquire psychomotor skills for endoscopic endonasal surgery using a personal webcam trainer: clinical article. J Neurosurg 118:1120–1126. https://doi.org/10.3171/2012.12.JNS12908
Jusue-Torres I, Sivakanthan S, Pinheiro-Neto CD, Gardner PA, Snyderman CH, Fernandez-Miranda JC (2013) Chicken wing training model for endoscopic microsurgery. J Neurol Surg Part B Skull Base 74:286–291. https://doi.org/10.1055/s-0033-1348026
Singh R, Baby B, Damodaran N, Srivastav V, Suri A, Banerjee S, Kumar S, Kalra P, Prasad S, Paul K, Anand S, Kumar S, Dhiman V, Ben-Israel D, Kapoor KS (2016) Design and validation of an open-source, partial task trainer for endonasal neuro-endoscopic skills development: Indian experience. World Neurosurg 86:259–269. https://doi.org/10.1016/j.wneu.2015.09.045
Sanromán-Álvarez P, Simal-Julián JA, García-Piñero A, Miranda-Lloret P (2017) Multitask box trainer for endoscopic endonasal skull base surgery: ENDOtrainer. World Neurosurg 101:304–307. https://doi.org/10.1016/j.wneu.2017.02.008
Berkowitz SS (2017) Teaching transnasal endoscopy to graduate students without a hospital or simulation laboratory: pool noodles and cadavers. Am J Speech Lang Pathol 26:709–715. https://doi.org/10.1044/2017_AJSLP-15-0119
Srivastav VK, Baby B, Singh R, Kalra P, Suri A (2017) Neuro-endo-trainer-online assessment system (NET-OAS) for neuro-endoscopic skills training, pp 213–219
Xie T, Zhang X, Gu Y, Sun C, Liu T (2018) A low cost and stepwise training model for skull base repair using a suturing and knotting technique during endoscopic endonasal surgery. Eur Arch Otorhinolaryngol 275:2187–2192. https://doi.org/10.1007/s00405-018-5024-2
Altun A, Cokluk C (2020) Endoscopic training model for intranasal transsphenoidal hypophysis surgery using a skull model and chicken wings. Turk Neurosurg 30:377–381. https://doi.org/10.5137/1019-5149.JTN.25841-19.4
Gallet P, Rebois J, Nguyen D-T, Jankowski R, Perez M, Rumeau C (2021) Simulation-based training in endoscopic endonasal surgery: assessment of the cyrano simulator. Eur Ann Otorhinolaryngol Head Neck Dis 138:29–34. https://doi.org/10.1016/j.anorl.2020.08.012
Tikka S, Chaithra BG, Sharma SB, Janakiram TN (2021) A feasible, low-cost, capsicum and tomato model for endoscopic sinus and skull base surgery training. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/s12070-021-02583-z
Bright RR, Varghese L, Kurien R (2021) Construct and validation of a three-dimensional physical model for training in transnasal office procedures. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/s12070-021-02775-7
Briner HR, Simmen D, Jones N, Manestar D, Manestar M, Lang A, Groscurth P (2007) Evaluation of an anatomic model of the paranasal sinuses for endonasal surgical training. Rhinology 45:20–23
Chen G, Ling F (2010) A new plastic model of endoscopic technique training for endonasal transsphenoidal pituitary surgery. Chin Med J (Engl) 123:2576–2579. https://doi.org/10.3760/cma.j.issn.0366-6999.2010.18.016
Okuda T, Kataoka K, Kato A (2010) Training in endoscopic endonasal transsphenoidal surgery using a skull model and eggs. Acta Neurochir (Wien) 152:1801–1804. https://doi.org/10.1007/s00701-010-0728-0
Waran V, Pancharatnam D, Thambinayagam HC, Raman R, Rathinam AK, Balakrishnan YK, Tung TS, Rahman ZA (2014) The utilization of cranial models created using rapid prototyping techniques in the development of models for navigation training. J Neurol Surg Part Cent Eur Neurosurg 75:12–15. https://doi.org/10.1055/s-0032-1330960
Okuda T, Yamashita J, Fujita M, Yoshioka H, Tasaki T, Kato A (2014) The chicken egg and skull model of endoscopic endonasal transsphenoidal surgery improves trainee drilling skills. Acta Neurochir (Wien) 156:1403–1407. https://doi.org/10.1007/s00701-014-2035-7
Chan HHL, Siewerdsen JH, Vescan A, Daly MJ, Prisman E, Irish JC (2015) 3D rapid prototyping for otolaryngology-head and neck surgery: applications in image-guidance, surgical simulation and patient-specific modeling. PLoS ONE 10. https://doi.org/10.1371/journal.pone.0136370
Narayanan V, Narayanan P, Rajagopalan R, Karuppiah R, Rahman ZAA, Wormald P-J, Van Hasselt CA, Waran V (2015) Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol 272:753–757. https://doi.org/10.1007/s00405-014-3300-3
Engel DC, Ferrari A, Tasman A-J, Schmid R, Schindel R, Haile SR, Mariani L, Fournier J-Y (2015) A basic model for training of microscopic and endoscopic transsphenoidal pituitary surgery: the Egghead. Acta Neurochir (Wien) 157:1771–1777. https://doi.org/10.1007/s00701-015-2544-z
Tai BL, Wang AC, Joseph JR, Wang PI, Sullivan SE, McKean EL, Shih AJ, Rooney DM (2016) A physical simulator for endoscopic endonasal drilling techniques: technical note. J Neurosurg 124:811–816. https://doi.org/10.3171/2015.3.JNS1552
Kashapov LN, Kashapov NF, Kashapov RN, Pashaev BY (2016) The application of additive technologies in creation a medical simulator-trainer of the human head operating field. IOP Conf Ser Mater Sci Eng 134:012011. https://doi.org/10.1088/1757-899X/134/1/012011
Wen G, Cong Z, Liu K, Tang C, Zhong C, Li L, Dai X, Ma C (2016) A practical 3D printed simulator for endoscopic endonasal transsphenoidal surgery to improve basic operational skills. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 32:1109–1116. https://doi.org/10.1007/s00381-016-3051-0
Shah KJ, Peterson JC, Beahm DD, Camarata PJ, Chamoun RB (2016) Three-dimensional printed model used to teach skull base anatomy through a transsphenoidal approach for neurosurgery residents. Oper Neurosurg Hagerstown Md 12:326–329. https://doi.org/10.1227/NEU.0000000000001127
Zheng J-P, Li C-Z, Chen G-Q, Song G-D, Zhang Y-Z (2018) Three-dimensional printed skull base simulation for transnasal endoscopic surgical training. World Neurosurg 111:e773–e782. https://doi.org/10.1016/j.wneu.2017.12.169
Masuda T, Omata S, Morita A, kin T, Saito N, Yamashita J, Chinzei K, Haswgawa A, Fukuda T, Kanako H, Adachi S, Arai F (2018) Patients Simulator for Transsphenoidal Surgery. In: 2018 International Symposium on Micro-NanoMechatronics and Human Science (MHS). 1–2. https://doi.org/10.1109/MHS.2018.8886922
Lin J, Zhou Z, Guan J, Zhu Y, Liu Y, Yang Z, Lin B, Jiang Y, Quan X, Ke Y, Xu T (2018) Using three-dimensional printing to create individualized cranial nerve models for skull base tumor surgery. World Neurosurg 120:e142–e152. https://doi.org/10.1016/j.wneu.2018.07.236
Ding C-Y, Yi X-H, Jiang C-Z, Xu H, Yan X-R, Zhang Y-L, Kang D-Z, Lin Z-Y (2019) Development and validation of a multi-color model using 3-dimensional printing technology for endoscopic endonasal surgical training. Am J Transl Res 11:1040–1048
Zheng J-P, Li C-Z, Chen G-Q (2019) Multimaterial and multicolor 3D-printed model in training of transnasal endoscopic surgery for pituitary adenoma. Neurosurg Focus 47:E21. https://doi.org/10.3171/2019.6.FOCUS19294
Shen Z, Xie Y, Shang X, Xiong G, Chen S, Yao Y, Pan Z, Pan H, Dong X, Li Y, Guo C, Wang F-Y (2020) The manufacturing procedure of 3D printed models for endoscopic endonasal transsphenoidal pituitary surgery. Technol Health Care Off J Eur Soc Eng Med 28:131–150. https://doi.org/10.3233/THC-209014
London NR, Rangel GG, VanKoevering K, Zhang A, Powell AR, Prevedello DM, Carrau RL, Walz PC (2021) Simulation of pediatric anterior skull base anatomy using a 3D printed model. World Neurosurg 147:e405–e410. https://doi.org/10.1016/j.wneu.2020.12.077
Masalha MA, VanKoevering KK, Latif OS, Powell AR, Zhang A, Hod KH, Prevedello DM, Carrau RL (2021) Simulation of cerebrospinal fluid leak repair using a 3-dimensional printed model. Am J Rhinol Allergy 19458924211003536. https://doi.org/10.1177/19458924211003537
Lai M, Skyrman S, Kor F, Homan R, El-Hajj VG, Babic D, Edström E, Elmi-Terander A, Hendriks BHW, de With PHN (2022) Development of a CT-Compatible, Anthropomorphic Skull and Brain Phantom for Neurosurgical Planning, Training, and Simulation. Bioengineering 9(10):537. https://doi.org/10.3390/bioengineering9100537
ProDelphus. http://www.prodelphus.com.br/websiteBR/website/home/. Accessed 23 Dec 2022
Neuroendoscopic Surgery Training Simulator – GTSimulators.com. https://www.gtsimulators.com/products/neuroendoscopic-surgery-training-simulator-snt. Accessed 3 Dec 2022
(2008) SIMONT ENT AND SKULL BASE SIMULATOR - Youtube. https://www.youtube.com/watch?v=iUqcVprRXuk. Accessed 23 Dec 2022
Nogueira JF, Stamm AC, Lyra M, Balieiro FO, Leão FS (2008) Building a real endoscopic sinus and skull-base surgery simulator. Otolaryngol Head Neck Surg 139:727–728. https://doi.org/10.1016/j.otohns.2008.07.017
Zymberg S, Vaz-Guimarães Filho F, Lyra M (2010) Neuroendoscopic training: presentation of a new real simulator. Minim Invasive Neurosurg MIN 53:44–46. https://doi.org/10.1055/s-0029-1246169
Japan Medical Company. In: Jpn. Med. Co. https://japanmedicalcompany.co.jp/en. Accessed 16 Mar 2023
3D Printed Medical Anatomy Models from JAPAN | KEZLEX. https://www.kezlex.com/en/. Accessed 5 Dec 2022
3D Printed Skull Base for Head Holder Model | A22 | KEZLEX. https://www.kezlex.com/en/products/skull/a22. Accessed 3 Dec 2022
3D Printed Endoscopic for Pituitary Gland Model | A39 | KEZLEX. https://www.kezlex.com/en/products/skull/a39/. Accessed 17 Nov 2021
3D Printed Internal Carotid Artery Injury ICAI Model Model | A43 | KEZLEX. https://www.kezlex.com/en/products/skull/a43/. Accessed 3 Dec 2022
Maza G, VanKoevering KK, Yanez-Siller JC, Baglam T, Otto BA, Prevedello DM, Carrau RL (2019) Surgical simulation of a catastrophic internal carotid artery injury: a laser-sintered model. Int Forum Allergy Rhinol 9:53–59. https://doi.org/10.1002/alr.22178
Muto J, Carrau RL, Oyama K, Otto BA, Prevedello DM (2017) Training model for control of an internal carotid artery injury during transsphenoidal surgery. Laryngoscope 127:38–43. https://doi.org/10.1002/lary.26181
Oyama K, Filho LFSD, Muto J, de Souza DG, Gun R, Otto BA, Carrau RL, Prevedello DM (2015) Endoscopic endonasal cranial base surgery simulation using an artificial cranial base model created by selective laser sintering. Neurosurg Rev 38:171–178. https://doi.org/10.1007/s10143-014-0580-4
Shen J, Wrobel BB, Zada G (2017) Management of vascular injuries during endoscopic skull base surgery: current strategies and simulation-based educational paradigms. Curr Otorhinolaryngol Rep 5:35–41. https://doi.org/10.1007/s40136-017-0146-4
Lin Q-S, Lin Y-X, Wu X-Y, Yao P-S, Chen P, Kang D-Z (2018) Utility of 3-dimensional–printed models in enhancing the learning curve of surgery of tuberculum sellae meningioma. World Neurosurg 113:e222–e231. https://doi.org/10.1016/j.wneu.2018.01.215
Wolfsberger S, Forster M-T, Donat M, Neubauer A, Bühler K, Wegenkittl R, Czech T, Hainfellner JA, Knosp E (2004) Virtual endoscopy is a useful device for training and preoperative planning of transsphenoidal endoscopic pituitary surgery. Minim Invasive Neurosurg MIN 47:214–220. https://doi.org/10.1055/s-2004-818523
Wolfsberger S, Neubauer A, Bühler K, Wegenkittl R, Czech T, Gentzsch S, Böcher-Schwarz H-G, Knosp E (2006) Advanced virtual endoscopy for endoscopic transsphenoidal pituitary surgery. Neurosurgery 59:1001–1009. https://doi.org/10.1227/01.NEU.0000245594.61828.41
Pöβneck A, Nowatius E, Trantakis C, Cakmak H, Maass H, Kühnapfel U, Dietz A, Strauβ G (2005) A virtual training system in endoscopic sinus surgery. Int Congr Ser 1281:527–530. https://doi.org/10.1016/j.ics.2005.03.184
Neubauer A, Wolfsberger S, Forster M-T, Mroz L, Wegenkittl R, Bühler K (2005) Advanced virtual endoscopic pituitary surgery. IEEE Trans Vis Comput Graph 11:497–506. https://doi.org/10.1109/TVCG.2005.70
Dixon BJ, Chan H, Daly MJ, Vescan AD, Witterick IJ, Irish JC (2012) The effect of augmented real-time image guidance on task workload during endoscopic sinus surgery. Int Forum Allergy Rhinol 2:405–410. https://doi.org/10.1002/alr.21049
Dixon BJ, Daly MJ, Chan H, Vescan A, Witterick IJ, Irish JC (2011) Augmented image guidance improves skull base navigation and reduces task workload in trainees: a preclinical trial. The Laryngoscope 121:2060–2064. https://doi.org/10.1002/lary.22153
Dixon BJ, Daly MJ, Chan H, Vescan A, Witterick IJ, Irish JC (2014) Augmented real-time navigation with critical structure proximity alerts for endoscopic skull base surgery. The Laryngoscope 124:853–859. https://doi.org/10.1002/lary.24385
Prisman E, Daly MJ, Chan H, Siewerdsen JH, Vescan A, Irish JC (2011) Real-time tracking and virtual endoscopy in cone-beam CT-guided surgery of the sinuses and skull base in a cadaver model. Int Forum Allergy Rhinol 1:70–77. https://doi.org/10.1002/alr.20007
De Notaris M, Topczewski T, De Angelis M, Enseñat J, Alobid I, Gondolbleu AM, Soria G, Gonzalez JB, Ferrer E, Prats-Galino A (2013) Anatomic skull base education using advanced neuroimaging techniques. World Neurosurg 79:S16.e9–S16.e13. https://doi.org/10.1016/j.wneu.2012.02.027
Varshney R, Frenkiel S, Nguyen LHP, Young M, Del Maestro R, Zeitouni A, Tewfik MA, Hovdebo J, Choudhury N, Debergue P, DeLuca G, Jiang D, Pazos V, Comas O, Neubauer A, Cabral A, Laroche D, Thibault F, DiRaddo R (2014) Development of the McGill simulator for endoscopic sinus surgery: a new high-fidelity virtual reality simulator for endoscopic sinus surgery. Am J Rhinol Allergy 28:330–334. https://doi.org/10.2500/ajra.2014.28.4046
NeuroVR – NeuroSim. https://neurosim.mcgill.ca/neurotouch. Accessed 5 Dec 2022
Li L, Yang J, Chu Y, Wu W, Xue J, Liang P, Chen L (2016) A novel augmented reality navigation system for endoscopic sinus and skull base surgery: a feasibility study. PloS One 11:e0146996. https://doi.org/10.1371/journal.pone.0146996
Won T-B, Hwang P, Lim JH, Cho S-W, Paek SH, Losorelli S, Vaisbuch Y, Chan S, Salisbury K, Blevins NH (2018) Early experience with a patient-specific virtual surgical simulation for rehearsal of endoscopic skull-base surgery. Int Forum Allergy Rhinol 8:54–63. https://doi.org/10.1002/alr.22037
CardinalSim. In: CardinalSim. https://med.stanford.edu/cardinalsim.html. Accessed 28 Feb 2023
Barber SR, Jain S, Son Y-J, Chang EH (2018) Virtual functional endoscopic sinus surgery simulation with 3D-printed models for mixed-reality nasal endoscopy. Otolaryngol - Head Neck Surg U S 159:933–937. https://doi.org/10.1177/0194599818797586
Heredia-Pérez SA, Harada K, Padilla-Castañeda MA, Marques-Marinho M, Márquez-Flores JA, Mitsuishi M (2019) Virtual reality simulation of robotic transsphenoidal brain tumor resection: evaluating dynamic motion scaling in a master-slave system. Int J Med Robot 15. https://doi.org/10.1002/rcs.1953
Lai M, Skyrman S, Shan C, Babic D, Homan R, Edström E, Persson O, Burström G, Elmi-Terander A, Hendriks BHW, de With PHN (2020) Correction: Fusion of augmented reality imaging with the endoscopic view for endonasal skull base surgery; a novel application for surgical navigation based on intraoperative cone beam computed tomography and optical tracking. PloS One 15:e0229454. https://doi.org/10.1371/journal.pone.0229454
Lai M, Skyrman S, Shan C, Babic D, Homan R, Edström E, Persson O, Burström G, Elmi-Terander A, Hendriks BHW, de With PHN (2020) Fusion of augmented reality imaging with the endoscopic view for endonasal skull base surgery; a novel application for surgical navigation based on intraoperative cone beam computed tomography and optical tracking. PLoS ONE 15:e0227312. https://doi.org/10.1371/journal.pone.0227312
Kim DH, Kim HM, Park J-S, Kim SW (2020) Virtual reality haptic simulator for endoscopic sinus and skull base surgeries. J Craniofac Surg 31:1811–1814. https://doi.org/10.1097/SCS.0000000000006395
Cai S, Zhou Y, Shen J, Guo J, Xiong X, Jiang X (2022) Augmented Reality Based Surgical Training and Education System for Neurosurgery,. 022 International Conference on Advanced Robotics and Mechatronics (ICARM). Guilin, China, pp 678–681. https://doi.org/10.1109/ICARM54641.2022.9959349
VOXEL-MAN Sinus. https://www.voxel-man.com/simulators/sinus/. Accessed 23 Dec 2022
UKE - UKE - Knowledge - Research - Healing. https://www.uke.de/english/. Accessed 23 Dec 2022
(2017) Virtual Infundibulotomy - Youtube. https://www.youtube.com/watch?v=Z0Y3-SX9Cvc. Accessed 23 Dec 2022
Tolsdorff B, Pommert A, Höhne KH, Petersik A, Pflesser B, Tiede U, Leuwer R (2010) Virtual reality: a new paranasal sinus surgery simulator. The Laryngoscope 120:420–426. https://doi.org/10.1002/lary.20676
Distributors. https://www.voxel-man.com/distributors/. Accessed 23 Dec 2022
Voxel-Man ENT Full System Virtual Reality Simulator. Anat. Wareh https://anatomywarehouse.com/voxel-man-ent-full-system-virtual-reality-simulator-A-112324. Accessed 16 Mar 2023
(2011) NeuroTouch: Transnasal Navigation - Youtube. https://www.youtube.com/watch?v=89A50YRy3gc. Accessed 16 Mar 2023
Rosseau G, Bailes J, Del Maestro R, Cabral A, Choudhury N, Comas O, Debergue P, De Luca G, Hovdebo J, Jiang D, Laroche D, Neubauer A, Pazos V, Thibault F, Diraddo R (2013) The Development of a virtual simulator for training neurosurgeons to perform and perfect endoscopic endonasal transsphenoidal surgery. Neurosurgery 73:S85–S93. https://doi.org/10.1227/NEU.0000000000000112
Simulation Based Medical Education Solutions | CAE Healthcare. https://www.caehealthcare.com/. Accessed 8 May 2023
Phacon – the patients for your surgical demonstration and education. https://www.phacon.de/en/. Accessed 17 Nov 2021
(2020) phacon sinus assistant gettingstarted endoscopy - Youtube. https://www.youtube.com/watch?v=Iy9QACSR6AY. Accessed 23 Dec 2022
(2021) Video Guide | PHACON Sinus Trainer - Youtube. https://www.youtube.com/watch?v=NDrKDvCdMuE. Accessed 23 Dec 2022
Stephenson ED, Farquhar DR, Masood MM, Capra G, Kimple A, Ebert CS, Thorp BD, Zanation AM (2019) Blinded evaluation of endoscopic skill and instructability after implementation of an endoscopic simulation experience. Am J Rhinol Allergy 33:681–690. https://doi.org/10.1177/1945892419860973
[S-00007] PHACON Sinus Assistant – Phacon. https://phacon.de/en/produkt/phacon-sinus-assistant-s-00003/. Accessed 22 Dec 2022
[S-00005] PHACON Sinus Trainer – Phacon. https://phacon.de/en/produkt/phacon-sinus-trainer-s-00005/. Accessed 22 Dec 2022
[SN-ah] PHACON Sinus Patient “Meyer” – pituitary tumor – Phacon. https://phacon.de/en/produkt/sn-ah-phacon-sinus-patient-meyer-hypophysen-tumor/. Accessed 8 May 2023
The evolution of Neurosurgery Learning | UpSurgeOn. https://www.upsurgeon.com/. Accessed 23 Dec 2022
TNS Box | Self-training System for endoscopic pituitary surgery - YouTube. https://www.youtube.com/watch?v=71-lGn5yIck. Accessed 23 Dec 2022
Newall N, Khan DZ, Hanrahan JG, Booker J, Borg A, Davids J, Nicolosi F, Sinha S, Dorward N, Marcus H (2022) High fidelity simulation of the endoscopic transsphenoidal approach: validation of the UpSurgeOn TNS Box. Front Surg 9. https://doi.org/10.3389/fsurg.2022.1049685
(2023) TNSBox – UpSurgeOn Store. https://store.upsurgeon.com/product/tnsbox/. Accessed 28 Feb 2023
Disposable Cavities – UpSurgeOn Store. https://store.upsurgeon.com/product/disposable-cavities/. Accessed 8 May 2023
Clarke DB, D’Arcy RCN, Delorme S, Laroche D, Godin G, Hajra SG, Brooks R, Diraddo R (2013) Virtual reality simulator: demonstrated use in neurosurgical oncology. Surg Innov 20:190–197. https://doi.org/10.1177/1553350612451354
Petrone S, Cofano F, Nicolosi F, Spena G, Moschino M, Di Perna G, Lavorato A, Lanotte MM, Garbossa D (2022) Virtual-augmented reality and life-like neurosurgical simulator for training: first evaluation of a hands-on experience for residents. Front Surg 9. https://doi.org/10.3389/fsurg.2022.862948
Zhang X-D, Li Z-H, Wu Z-S, Lin W, Lin W-J, Lin J-C, Zhuang L-M (2018) A novel three-dimensional-printed paranasal sinus–skull base anatomical model. Eur Arch Otorhinolaryngol 275:2045–2049. https://doi.org/10.1007/s00405-018-5051-z
Huang X, Fan N, Wang H-J, Zhou Y, Li X, Jiang X-B (2021) Application of 3D printed model for planning the endoscopic endonasal transsphenoidal surgery. Sci Rep 11:5333. https://doi.org/10.1038/s41598-021-84779-5
Huang X, Liu Z, Wang X, Li X-D, Cheng K, Zhou Y, Jiang X-B (2019) A small 3D-printing model of macroadenomas for endoscopic endonasal surgery. Pituitary 22:46–53. https://doi.org/10.1007/s11102-018-0927-x
Panesar SS, Magnetta M, Mukherjee D, Abhinav K, Branstetter BF, Gardner PA, Iv M, Fernandez-Miranda JC (2019) Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg Focus 47:E12. https://doi.org/10.3171/2019.9.FOCUS19511
Chaudhary A, Chopra S, Sinha VD (2021) Role of three-dimensional printing in neurosurgery: an institutional experience. Asian J Neurosurg 16:531–538. https://doi.org/10.4103/ajns.AJNS_475_20
Chopra S, Boro AK, Sinha VD (2021) 3D printing-assisted skull base tumor surgeries: an institutional experience. J Neurosci Rural Pract 12:630–634. https://doi.org/10.1055/s-0041-1734001
Digital future | Bracco. https://www.bracco.com/en-us/digital-future. Accessed 15 Mar 2023
de Notaris M, Palma K, Serra L, Enseñat J, Alobid I, Poblete J, Gonzalez JB, Solari D, Ferrer E, Prats-Galino A (2014) A three-dimensional computer-based perspective of the skull base. World Neurosurg 82:S41–S48. https://doi.org/10.1016/j.wneu.2014.07.024
Wang S, Zhang S, Jing J (2012) Stereoscopic virtual reality models for planning tumor resection in the sellar region. BMC Neurol 12:146. https://doi.org/10.1186/1471-2377-12-146
Wang S-S, Xue L, Jing J-J, Wang R-M (2012) Virtual reality surgical anatomy of the sphenoid sinus and adjacent structures by the transnasal approach. J Cranio-Maxillofac Surg 40:494–499. https://doi.org/10.1016/j.jcms.2011.08.008
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Francesco Doglietto had the idea for the article. Material preparation, data collection, and analysis were performed by Giacomo Santona and Alba Madoglio. The first draft of the manuscript was written by Giacomo Santona, Alba Madoglio, and Francesco Doglietto and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
No ethical approval is required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Santona, G., Madoglio, A., Mattavelli, D. et al. Training models and simulators for endoscopic transsphenoidal surgery: a systematic review. Neurosurg Rev 46, 248 (2023). https://doi.org/10.1007/s10143-023-02149-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02149-3