Abstract
Chronic pain constitutes one of the most common chronic complaints that people experience. According to the International Association for the Study of Pain, chronic pain is defined as pain that persists or recurs longer than 3 months. Chronic pain has a significant impact on individuals’ well-being and psychosocial health and the economy of healthcare systems as well. Despite the availability of numerous therapeutic modalities, treatment of chronic pain can be challenging. Only about 30% of individuals with non-cancer chronic pain achieve improvement from standard pharmacological treatment. Therefore, numerous therapeutic approaches were proposed as a potential treatment for chronic pain including non-opioid pharmacological agents, nerve blocks, acupuncture, cannabidiol, stem cells, exosomes, and neurostimulation techniques. Although some neurostimulation methods such as spinal cord stimulation were successfully introduced into clinical practice as a therapy for chronic pain, the current evidence for brain stimulation efficacy in the treatment of chronic pain remains unclear. Hence, this narrative literature review aimed to give an up-to-date overview of brain stimulation methods, including deep brain stimulation, motor cortex stimulation, transcranial direct current stimulation, repetitive transcranial magnetic stimulation, cranial electrotherapy stimulation, and reduced impedance non-invasive cortical electrostimulation as a potential treatment for chronic pain.
Similar content being viewed by others
Introduction
Chronic pain constitutes one of the most common chronic complaints that people experience, with the prevalence rate ranging between 11% and 40% in the USA [1]. A recent epidemiological study demonstrated that more than 1 in 5 Americans suffer from chronic pain [2], whereas in Europe chronic pain affects about 19% of the adult population with the highest prevalence in Poland and Norway (27% and 30% respectively) [3].
According to the International Association for the Study of Pain (IASP), chronic pain is defined as pain that persists or recurs longer than three months [4]. Patients suffering from chronic pain may experience a drop in their quality of life due to social dysfunctions, sleep disorders, and depression [5, 6]. Moreover, chronic pain has a significant impact not on individuals’ well-being only but also on the economy of healthcare systems. In the USA, estimated economic costs of chronic pain range between $560 and $635 billion annually, including direct healthcare costs and lost productivity [7].
A recently published classification of chronic pain designed by the IASP for the 11th Edition of the International Classification of Diseases (ICD-11) divides chronic pain into chronic primary pain and chronic secondary pain [8]. While the primary pain is related to remarkable emotional distress and/or functional dysfunction without other known causes [8], the secondary pain is a result of an underlying condition such as tumors, injury, surgery, musculoskeletal disease, or nerve damage [9,10,11,12,13,14,15].
Despite the availability of numerous therapeutic modalities, the treatment of chronic pain can be challenging. Conventional management of this condition includes oral analgesics as the first-line treatment administered according to the World Health Organization analgesic ladder [16]. However, for cancer-related chronic pain, this low-cost and simple therapeutic tool is effective in 75–90% of patients, only about 30% of individuals with non-cancer chronic pain achieve improvement from opioid treatments [17, 18]. Considering the possible side effects of long-term opioid use (78% overall adverse event rate, including 7.5% serious adverse events) and the risk of addiction, the harms outweigh the benefits of opioid therapy [19]. For this reason, in treatment-resistant cases, dose reduction or discontinuation of opioid treatment may be considered towards alternative therapies [20, 21]. Numerous therapeutic approaches were proposed as a potential treatment for chronic pain including non-opioid pharmacological agents, nerve blocks, acupuncture, cannabidiol, stem cells, exosomes, and neurostimulation techniques [22,23,24,25,26,27]. Moreover, the role of the psychosocial aspect in chronic pain treatment and the multimodality of therapy is emphasized by recent reports [28,29,30,31].
Even though the idea of neurostimulation originated more than a century ago, this therapeutic modality grew the attention of researchers only in the last decades [32]. Numerous studies demonstrated neurostimulation techniques as a potential treatment for a variety of neurological disorders such as epilepsy, Parkinson’s disease, dystonia, and many others [33,34,35,36]. Among developed neurostimulation methods, three major groups can be distinguished—brain stimulation, spinal cord stimulation (SCS), and peripheral nerve stimulation [37,38,39,40]. Although the clinical use of SCS was recently approved by the Food and Drug Administration as a therapy for chronic pain [41, 42], the current evidence for brain stimulation efficacy in the treatment of chronic pain remains unclear.
Hence, this narrative literature review aimed to give an up-to-date overview of brain stimulation methods, including deep brain stimulation (DBS), motor cortex stimulation (MCS), transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), cranial electrotherapy stimulation (CES), and reduced impedance non-invasive cortical electrostimulation (RINCE), as regards their mechanism of action, clinical efficacy, and common adverse effects, in the treatment of chronic pain.
Materials and methods
This narrative review was conducted according to the Scale for the Assessment of Narrative Review Articles (SANRA) criteria [43]. A literature search was performed in November and December 2022 based on the MEDLINE (PubMed) database with the use of the following terms: “neurostimulation,” “brain stimulation,” “chronic pain,” “pain,” “deep brain stimulation,” “motor cortex stimulation,” “transcranial direct current stimulation,” “repetitive transcranial magnetic stimulation,” “cranial electrotherapy stimulation,” “reduced impedance non-invasive cortical electrostimulation” (Fig. 1)
The literature search was limited to articles published in the last 10 years (between January 2013 and December 2022) to provide current data and trends in therapeutic outcomes of brain stimulation. Moreover, a comprehensive search of selected papers’ references was conducted to identify further articles of interest. Special attention was devoted to clinical trials. Case reports, retrospective studies, and articles written in languages other than English were excluded.
Brain stimulation methods for chronic pain
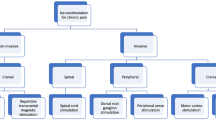
Among discussed techniques of brain stimulation, invasive as well as non-invasive methods can be distinguished. Non-invasive techniques are mainly based on the percutaneous stimulation system and include techniques such as tDCS, rTMS, CES, and RINCE. On the other hand, invasive methods require neurosurgical procedures for electrode implantation and include methods such as DBS and MCS (Fig. 2).
Graphical presentation of available brain stimulation techniques. Parts of the figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/)
Invasive brain stimulation
Deep brain stimulation
DBS is a method of delivering an electric current to the brain through implanted electrodes. It uses high stimulation frequencies that functionally deactivate the neurons present near the electrodes but the fiber pathways can still be stimulated [44].
The most common DBS targets in chronic pain treatment include the periventricular and periaqueductal gray matter (PVG/PAG) and the rostral anterior cingulate cortex (ACC). The choice of simulation target depends on the cause of the present pain. For example, the best approach for stimulation for phantom limb pain is thalamic DBS, which was supported by results of pain reduction ranging from 50.6 to 76.4% in patients participating in the study conducted by Abreu et al. [45]
Drug-refractory chronic cluster headache is among the clinical conditions where the application of DBS may be efficient. The usage of high-frequency discharges has been proven to reduce the occurrence of complaints in 60% of patients, with up to 30% of patients having their pain completely relieved [46]. The location that turns out to be the pulse generator in this type of discomfort appears to be the area between the hypothalamus and the mesencephalon. An alternative technique to the mentioned above is invasive sphenopalatine ganglion stimulation. The method of action involves parasympathetic inhibition using high-frequency stimulation [47]. The success of this method was confirmed by a randomized multicenter study, according to which pain reduction was achieved in 67% of patients with cluster headaches and complete absence of pain occurred in 34% of treated individuals [48]. The results after 24 months showed a pain reduction of 65%, and more than 50% decrease in the frequency of attacks was achieved in 43% of patients. This suggests that using this type of stimulation may serve as a preventive treatment for chronic cluster headaches [47].
What seems noteworthy is the hypothesis of an endogenous opioid secretion mechanism when the brain is stimulated within the PAG. A study was conducted on a sample of five patients with the DBS system implemented. The researchers used the opioid radiotracer [11C]-diprenorphine (DPN). This compound belonging to the opioid receptor agonists showed high levels of binding in the thalamus, midbrain, and several cortical regions but low levels of binding in the occipital cortex and the pontine nucleus [49]. The results seem to suggest the secretion of endogenous opioids, which prevented DPN attachment. However, despite these reports, which have not been fully confirmed, the exact mechanism remains to be determined. Nevertheless, the potential for disorienting psychoactive, respiratory depression, and other side effects in chronic pain patients who manage only with opioid drugs may be the impetus for an in-depth discovery of the mechanisms at play here and thus originate novel effective and tolerable treatment paradigms to lessen the suffering of patients [50].
Research into the effects of DBS has resulted in the identification of a new anatomical target that appears to be a combination of the sensory and limbic systems, which takes place in a situation of painful sensations. This target turns out to be the posterior insula due to its role as a link between the spinothalamic pathway and the ventroposteromedial nucleus of the thalamus [51]. Also confirming the need to consider this point seems to be the fact that functional imaging has demonstrated activation of the posterior insula on individuals to a level dependent on pain intensity, as well as the phenomenon of neutrality to painful stimuli occurring in patients whose brain lesion has involved the insula.
Considering the hypothesis according to chronic pain results from faulty synchronization between brain networks encoding the somatosensory, affective, and cognitive impulses, DBS seems to be an ideal source of a mechanism to interrupt this severe synchrony [52]. The idea of closed-loop stimulation requires a device that reads brain waves of specific frequency bands from three areas of the brain (primary somatosensory cortex, the dorsal anterior cingulate, and the orbitofrontal cortex) and then determines the signals recognized as a baseline based on them. The device should be able to interpret the stronger signals and generate pulses to interrupt the disturbance experienced as pain. An alternative to this solution can be open-loop stimulation, the difference of which is the absence of the presence of sensors, so in this solution, decoupling of neural signals occurs all the time, or patient-triggered stimulation, in which the patient controls the device, but in this case, ideally, the pain signals should be of somatosensory origin [52].
Although the clinical studies demonstrated favorable results of applying the DBS for chronic pain treatment (Table 1), the number of patients treated by this method is declining. Lack of approval for the clinical use of this method in some countries and improvement of the other treatment approaches are some of the many factors responsible for that phenomenon. Despite that, patients with severe, exhausting neuropathic pain with objective pathology refractory to more conservative treatments seem to be the best candidates for this method [44].
DBS presents the risks of open surgical procedures, including severe complications such as hemorrhages and infections. The concern may also be triggered by the possibility of a stimulation-induced seizure [58].
Depending on the location of the electrodes, symptoms such as paresthesias, muscle spasms (stimulation near the internal pouch), and phosphenes (stimulation near the optic nerve) can be expected. Side effects can also occur with using ordinary therapeutic voltages, but then it is necessary to reposition the electrode in a slightly altered location [59].
Motor cortex stimulation
MCS is an invasive neurostimulation method proposed as an alternative treatment for chronic neuropathic pain refractory to the standard therapy [60]. First reports regarding the successful clinical use of MCS for chronic pain date from the early 1990s and concern the treatment of chronic thalamic pain [61]. So far, MCS was investigated in numerous clinical studies as a potential treatment for various chronic pain conditions including central post-stroke pain (CPSP) as well as brachial plexus avulsion, trigeminal neuropathic pain, post-surgical pain, pain after spinal cord injury, and more [62].
Implantation of an MCS stimulator involves craniotomy and placement of the electrode in the part of the precentral gyrus or central sulcus corresponding to the painful area. Based on the anatomical spaces where the stimulator leads can be placed, there exist two types of MCS—subdural MCS and epidural MCS [63]. Although studies did not demonstrate significant differences as regards clinical efficacy between these types of MCS [64], placement of the lead subdurally may be more reasonable in case of significant distance between cortex and dura mater [65].
In MCS, pain reduction is obtained by stimulating the region of the motor cortex appropriate for the painful area reported by the patient [62]. However, the exact mechanisms of action through which MCS relieves pain remain not fully understood. A few hypotheses were proposed to explain the analgesic effects of MCS. One of them concerns the modulation of analgesic pathways in the central nervous system by MCS [66]. Preclinical research initially demonstrated that MCS indirectly activates descending inhibitory pathway through a decrease of thalamic activity and in consequence activation of midbrain periaqueductal gray neurons [67]. The role of the ACC was also considered significant for the development of chronic pain [68]. A recent study demonstrated that ten daily sessions of MCS on the neuropathic pain animal model decreased mechanical allodynia and induced neuronal changes in ACC [69], whereas inhibition of protein kinase M zeta (PKMζ) hampered the effectiveness of MCS. Thus, activation of PKMζ in the ACC may be beneficial to the analgesic properties of MCS. The release of endogenous opioids induced by MCS was suggested as another factor responsible for its analgesic effect [70]. Moreover, it has been proposed that MCS modulates the descending analgesic pathways through the cannabinoid and opioid systems [71]. Therefore, compromising of spinal CB2 cannabinoid receptor activation in some groups of patients may be the cause of opioid as well as MCS resistance. Poor understanding of the mechanism underlying MCS-induced analgesia may be one of the causes contributing to refractory to this therapy in two third of patients with chronic pain [72]. Hence, research in this field should be continued to increase the clinical efficacy of MCS. Clinical trials conducted to date showed inconsistent results (Table 2). Moreover, most of them recruited a limited number of patients and were performed with low-quality methodology. Only four randomized controlled trials (RCTs) have been registered in the years between 2013 and 2022 [73, 78, 81, 85]. Some recent studies demonstrated significant chronic pain reduction in the majority of patients at long-term follow-up [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] [78, 81, 85, 79, 83]. In other trials, about 30–40% of patients experienced successful outcomes of chronic pain treatment by MCS at several-year follow-up [75]. However, some research showed unsuccessful outcomes or even stopped before complete data collection due to high complication rates and poor treatment results [81]. In a recent RCT, 39% of patients successfully responded to MCS [73]. However, adverse events were common in this study and concerned the majority of patients. Another RCT demonstrated the long-term benefits of MCS in half of the patients with follow-up ranging from 2 to 9 years [85]. Low rates of clinical efficacy and inconsistent results of available studies may suggest that MCS is beneficial for only specific subgroups of patients with chronic pain. Indeed, some reports suggested that MCS is more effective in the treatment of chronic pain associated with phantom limb pain, facial pain, and complex regional pain syndrome (CRPS) than in the treatment of CPSP or chronic pain resulting from brachial plexus avulsion [44, 73, 88]. On the other hand, in some of the recent studies, the use of MCS achieved significant improvements in patients with chronic pain caused by CPSP and trigeminal neuropathic pain [64, 79, 82, 89]. However, many other neurostimulation approaches have been studied in the treatment of facial pain [90]. One of them—stimulation of the Gasserian Ganglion—resulted in successful pain reduction in 44% of patients with refractory trigeminal neuropathy at 24-month follow-up [91]. Another neuromodulation technique—peripheral nerve field stimulation—provided satisfactory pain relief in 4 of 8 patients with trigeminal neuralgia associated with multiple sclerosis after 24 months of follow-up [92]. Despite their low evidence, these findings decrease the value of MCS in the treatment of facial pain syndromes considering its higher invasiveness. However, studies directly comparing the safety and performance of these methods with MCS in the treatment of facial pain have not been conducted to date.Considering other factors which may influence MCS effectiveness, some authors indicated that visual analogue scale at 1-month post-implantation and successful preoperative rTMS may be potential predictive factors of MCS [85, 89, 76]. Moreover, according to a recent systematic review, the younger age of patients with facial pain was positively related to the definitive MCS implantation rate [93]. Nevertheless, due to the lack of high-quality evidence, further studies should explore predictive factors for successful MCS to establish appropriate indications for this treatment method. As an invasive method, MCS is burdened with a high risk of complications. According to a recent systematic review, the most common adverse events include temporary partial seizures (18%) and wound infections (12%) [89]. In recent studies, epidural hematomas and dural scars have been also observed [79, 87]. Moreover, device failures such as electrode shifts or generator malfunctioning were present in a majority of studies from the last ten years reporting adverse events.
Non-invasive brain stimulation
Transcranial direct current stimulation
tDCS is a pioneering non-invasive method, constantly being improved by current research. The application of this method is diverse. Apart from chronic pain described in this work [94], tDCS has already been used in patients with various neuropsychiatric conditions such as depression, mania, schizophrenia, obsessive-compulsive disorder, panic, and post-traumatic stress disorder [95,96,97,98]. In addition, recent studies have shown that tDCS can also achieve positive results in Alzheimer’s disease treatment [99]. Additionally, tDCS can be used combined with techniques such as transcranial magnetic stimulation (TMS), fMRI, and electroencephalogram (EEG) to study how stimulation modulates cortical excitability [100]. It has proven to be safe, portable, and cost-effective [101].
Regarding the tDCS mechanism of action, it involves modulating the activity of the brain which is conditioned by the supply of a small amplitude (usually no more than 2 mA) for a short period (from 10 to 30 min). Typically, the power source used for this test is a battery with two electrodes: an active (polarizing) and a reference electrode. They work according to basic neurophysiology. If the active electrode is an anode, it stimulates the motor cortex to become more excitable. If the cathode is the active electrode, the excitability of the motor cortex is reduced. It has been observed that when anode current is used, connection to the cortical region under the target electrode is easier. On the other hand, when cathode current is used, connection to the cortical region of the brain is inhibited. This is because the placement of the electrodes is as follows: at least one of the electrodes is placed on the scalp—it is through it that electronic currents, causing the polarization of neuronal cell membranes, pass through the skull to reach the brain, thanks to which the level of stimulation of the cortex increased or decreased [94]. The 10–20 EEG system can be applied to determine the location of the electrodes. Most often, the arrangement of the electrodes is in the supraorbital area. However, some studies showed that different localizations of the electrodes may affect the quantity and quality of the current delivered to the brain, which results in different intensities of stimulation delivered to the brain [102].
It should be emphasized that the size and shape of the placed electrodes are essential for successful stimulation. The density of the flowing current depends on this, which determines the total stimulation dose that can be administered to the subject. So far, according to current literature, the safe dose value cannot exceed 216 C/cm2 [103]. The most commonly used electrodes range in size from 25 to 35 cm2 (5 × 5 cm and 5 × 7 cm) [94]. When it comes to allowing the current to pass through the cerebral cortex, saline is usually used for this. It is recommended to use small saline containers (e.g., 20-ml bottles) that allow a little control of the amount of liquid applied to the sponges [94]. Alternatively, an electroconductive gel (such as EEG paste) can be used. It is applied to the base of the rubber electrode, so there is no need for sponge bags as with the use of saline. However, the gel can also dry quickly due to the temperature emitted by the electrode, which increases the risk of scalp burns [94].
The use of tDCS in the treatment of chronic pain has also been repeatedly described in medical literature. It gave the expected effects of reducing chronic pain when used in cases such as knee osteoarthritis [104], treating joint pain as chikungunya virus complication [105], several times in the treatment of fibromyalgia pain [106, 107], and treatment of multiple sclerosis [108]. However, in some studies, tDCS did not produce the expected results. In the study by Luedtke et al., five-day stimulation did not reduce disability associated with non-specific chronic low back pain [109]. O’Neill et al. also failed to prove that tDCS would be effective in patients with neuropathic pain [110]. Research is also underway on the use of tDCS as a method to inhibit neural changes caused by chronic stress. tDCS would then be used before the patient is exposed to chronic stress, which in the future may lower the pain threshold and lead to hyperalgesia [111].
Several RCTs have been registered using tDCS (Table 3). The results varied depending on the electrodes’ position, stimulation duration, and current intensity. Fregni et al. reported pain reduction after 16 days of active tDCS, and in another study, they increased the pain reduction effect to 21 days [128, 129]. Boggio et al. scored up to 28 days of pain relief [130]. Then, Mori et al. received a pain reduction effect lasting up to 28 days [131]. Soler et al. extended the effect of reducing pain up to 12 weeks [132]. As we can see, research on the treatment of pain using tDCS was conducted years ago and already had satisfactory results. Recently, De Souza et al. conducted a study on 58 women in the chronic phase of CHIK disease [105], with an average age of 52.85 years. This group was randomly divided into two: an active group (active-tDCS)—M1-S0—2 mA in a 20-min session was used, and a sham group (sham-tDCS). VAS and Brief Pain Inventory were used to assess pain, and functional capacity was assessed using a Health Assessment Questionnaire. After analyzing the results, six non-consecutive active tDCS sessions on M1 significantly reduced chronic joint pain associated with CHIK. However, no change in functional capacity was noted in any patients. People with epilepsy, metal implants in the stimulation sites, a history of alcohol abuse, breastfeeding women, and pregnant women were excluded from the study. An analysis of the cumulative percentage of respondents [105] showed that 79.31% of active-tDCS participants had a VAS score improvement of more than 30% compared to sham-tDCS. NNT (number needed to treat) calculated in this study was 2, which means two patients had to undergo this technique for one more to have the desired effect. Moreover, anodal tDCS applied for approximately 15–20 min significantly reduced phantom limb pain in amputees [133].
A recently updated version of the original Cochrane systematic review on the effectiveness of the tDCS method in the treatment of chronic pain [134] considered 747 participants (22 studies involved) and showed that pain intensity as measured by a visual analog scale was reduced by 17% with this that the quality of the evidence was assessed as very low, meaning that the results are likely to be significantly different from the estimated effect. A meta-analysis of the tDCS studies compared to the sham quality of life (measured using different scales in included studies) in the short term showed a positive effect (SMD 0.66 95% CI 0.21 to 1.11, low-quality evidence). tDCS may have short-term effects on chronic pain and quality of life, but multiple sources of bias may have contributed to the observed effects.
Although the use of the tDCS method has reported not many adverse events during many years of research, it has been discovered that there may be mild but also temporary side effects, such as headache, itching of the skin in places of stimulation, moderate fatigue, reddening of the skin under the electrode, difficulty concentrating, severe mood swings, and nausea [94]. As we can see, however, the side effects are insignificant compared to the achieved outcomes of therapy. It was even recognized that symptoms such as moderate fatigue could be related to participation in the experiment, and might not necessarily result from the effect of the tDCS method itself on the body [94]. The most common side effect reported by the respondents is skin itching, and although it usually disappears after the current stabilizes, methods have been developed to alleviate this effect [135].
Namely, the application of a moderate saline solution to the storage bag, using the increase/decrease procedure when tDCS is turned on or off, and the use of a smaller size of the electrodes significantly reduce the itching effect of the skin at the stimulation sites. However, these discounts should not be abused because, for example, when using electrodes of small size, the cost of the method may increase due to the need for a change of density and amount of current. While the research was conducted to monitor potential side effects, an adverse reaction questionnaire was published [136]. The questionnaire covered the 10 most common ailments: headache, neck pain, scalp pain, tingling, itching, burning sensation, redness of the skin, drowsiness, problems with concentration, and mood changes. Each item required participants to answer the question “Do you experience any of the following symptoms or side-effects?” on a 4-point scale: 1 = absent, 2 = mild, 3 = moderate, 4 = severe. However, since the publication of this questionnaire, only a few research groups have used it [137].
Moreover, regarding the side effects of tDCS therapy, special attention should be paid to the often overlooked issue. Namely, it is possible to direct the current towards important body areas, including the heart, respiratory system, and autonomic regions of the brainstem. During the initial tDCS experiments, it was noted that one participant experienced a brief episode of respiratory depression during stimulation when the electrode was placed outside the cerebral leg [138]. However, it used a current of 3 mA, a value above the current safety threshold of 2 mA. Therefore, to maximize the safety of this method, it is crucial to follow the correct current values.
Repetitive transcranial magnetic stimulation
TMS is a non-invasive treatment used to address certain neurological and psychological disorders (such as Parkinson’s disease, Alzheimer’s disease, and depression), by inducing depolarization of the neurons in the brain [139,140,141]. This is achieved by transmitting a strong current through a wire from a machine to a circular wire, which initiates a magnetic field in a perpendicular direction based on Faraday’s law of induction. When the circular wire is applied to the scalp, the time-changing magnetic field induces the current in the axons beneath the scalp, running in the opposite direction to the current in the coil, thereby stimulating the brain tissue [142].
There are 3 main types of TMS: single-pulse TMS, paired-pulse TMS, and trains of repetitive stimuli (repetitive TMS, or rTMS). In this article, we will only focus on rTMS. Typically, a single-pulse TMS lasts for only a few seconds. Therefore, to prolong its effects, repetitive TMS is used. rTMS works by firing the single-pulse stimuli repeatedly at a specific frequency, intensity, and time duration, either to inhibit or to stimulate the activity of a specific cortical area that is applied [143]. In rTMS, two different subgroups can be used for treatment based on their purpose. One is high-frequency rTMS (HF-rTMS), with frequencies ≥ 5 Hz, whereas the other is low-frequency rTMS (LF-rTMS), with frequencies ≤ 1 Hz. HF-rTMS can increase cortical excitability, and MEP size (i.e., motor-evoked potential, electric potential in the motor pathway induced by TMS) [144], and provoke intercellular interactions. As a result, it can enhance and facilitate cell proliferation, focal cerebral blood flow, and synaptic plasticity [142].
In studies of patients with neuropathic pain following a stroke, it has been shown that rTMS may help reduce pain by activating inhibitory pathways and stimulating neurogenesis [142]. rTMS has a range of applications, including post-stroke recovery [145], depression (especially major depression and treatment-resistant depression) [146], fibromyalgia [147], and other neuropathic pains [47].
The mechanism of rTMS on living animals is complicated, yet fascinating. This includes its effects on oligodendrocytes, astrocytes, microglia, and stem cells, which can result in the improvement of survival and maturation in oligodendrocyte stem cells, and the enhancement of neuronal metabolic activity and plasticity in astrocytes [142]. Although many studies proved the efficacy of TMS in treating depression and other psychological problems, the evidence supporting the use of rTMS for pain relief is not strong enough. Additionally, the mechanism of rTMS in reducing neuropathic pain remains unclear.
However, astrocytes have been suggested to play an essential role in suppressing pain. They help form the blood-brain barrier, which acts as a border wall protecting the brain from pathogens and allowing only certain amounts of small molecules, ions, and nutrients to move across the wall. Astrocytes also regulate the metabolism of neurons, phagocytose synapses, and remove debris. The inflammatory molecules, including neuronal nitric oxide synthase, glial acidic fibrillary acidic proteins, and 5-bromo-2-deoxyuridine, are released in astrocytes during the inflammatory process [142, 148]. The levels of these inflammatory agents are downregulated with the use of HF-rTMS (20Hz) treatment, which indicates that the technology can suppress the release of inflammatory substances and thus reduce neuropathic pain. In addition to the anti-inflammatory benefits, an increase in the level of anti-inflammatory mediator IL-10 has been observed after HF-rTMS (10 Hz) treatment, leading to greater neuronal plasticity and recovery after neuronal damage [142].
Thalamus is responsible for relaying and processing pain signals before transmitting them to the cerebral cortex. Some studies suggest that HF-rTMS may influence the activity of the cortical thalamic tract and inhibit the spinothalamic pathway in the ascending pathway and thalamic nuclei. The spinothalamic tract can be divided into two parts: the anterior spinothalamic tract, which is responsible for crude touch and pressure sensation, and the lateral spinothalamic tract, which carries sensations of pain and temperature. By inhibiting the ascending tract, HF-rTMS can reduce the transmission of nociceptive signals to the cerebral cortex and thalamus, thereby reducing the sensation of pain [148].
So far, all the research is only involved a limited number of people, which restricted the accountability of using rTMS to treat patients with chronic pain. However, it is still worth examining previous research (Table 4). Several studies have targeted MCS, which has shown greater pain reduction with longer session durations and more frequent stimulation using HF-rTMS [160]. In the results of four studies [149, 157, 158, 156], the levels of pain reduction are shown to be at least 40% of relief (a clinically significant reduction in pain intensity is approximately 30%), which implies the potential clinical value of the treatment in neuropathic pain. Although it appears to be clinically relevant, the small number of volunteers and a small number of sessions (5–10) and a short period of follow-up (<3 weeks) make it unreliable to put the technique into clinical practice. Thus, more patients need to be involved in the research, and longer sessions and durations of follow-up are required.
In one of the recent studies, HF-rTMS was administered to 36 patients with chronic central neuropathic pain in motor cortex M1, with 2 randomized phases spaced 3 weeks apart. Each phase included 4 consecutive rTMS sessions and 1 final evaluation session. The final results showed a significant analgesic effect in the active phase with a 33.8% confidence interval (CI), compared to the sham phase with only 13.02% CI. And no side effect effects were observed [161, 162].
Another research showed a significant pain reduction after repeatedly applying HF-rTMS to the primary cortex M1 for 25 weeks [162]. Interestingly, the reduction in pain intensity was not apparent after the initial 5 daily rTMS sessions but was observed after 4 weeks of treatment with eight rTMS sessions. This resulted in significant pain relief over the 25 weeks of rTMS treatment. At week 25, which is 3 weeks after the last rTMS session, the percentage of patients who completed the treatment and experienced more than a 50% reduction in pain was 44.7% for M1-rTMS and 12% for sham-rTMS. These results demonstrated the clinical relevance of rTMS in treating neuropathic pain. In addition, during the first 4 weeks of the treatment, patients reported an improvement in fatigue from their diaries, indicating that rTMS may also help alleviate chronic fatigue caused by neuropathic pain.
A large number of studies were conducted on rTMS from 2013 to 2022. Based on these studies, the most serious adverse effect is seizures; other than that, hearing impairment and short-term decline in cognition were reported, while minor side effects are local pain, headache, and discomfort. However, the occurrence of side effects is rare. Most patients who received TMS treatment did not experience unpleasant side effects, which suggests that it is safe to use in treating various disorders, including chronic neuropathic pain and major depressive disorder [163].
From the numbers of the studies, it has found that anyone with any condition who undergoes rTMS delivery may be at risk of TMS-induced seizures, ranging from healthy individuals to those with neurological (e.g., post-stroke, multiple sclerosis, traumatic brain injury, meningoencephalitis, and brain tumors) or psychiatric disorders (e.g., major depression, schizophrenia, bipolar disorder, dementia, and alcohol abuse). Certain factors have been suggested to increase the chance of TMS-induced seizures. For instance, certain medications and medical conditions that lower the seizure threshold may increase the chance of seizures caused by TMS stimulation. In theory, first-degree relatives of persons with epilepsy may also have a higher possibility of TMS-related seizures, yet no such event has been observed and reported so far. Apart from the previously mentioned risk factors, sleep deprivation is considered to be of particular relevance. Some studies have reported an increase in cortical excitability, which was monitored with EEG during TMS stimulations. The same results were found in healthy individuals as well.
Although hearing loss is categorized as one of the risk factors, it is usually due to loose ear plugs when rTMS is being administered. Some individuals whose ear plugs slipped out during the procedure have experienced transient increases in auditory thresholds; therefore, hearing safety measures should be taken seriously. In terms of cognitive TMS effects, experimental studies have observed short-term cognitive decline, with degrees of decline generally considered low to moderate. However, in clinical studies, no cognitive changes were found. Despite no evidence suggesting that TMS leads to cognitive impairment. Therefore, it is recommended that further studies should be conducted to evaluate the long-term impact of TMS on cognition.
Cranial electrotherapy stimulation
The idea of CES was initially developed in Russia in the 1950s as a therapy for anxiety, depression, and insomnia, to be later used in pain treatment [134, 164]. The first device was called the Somniatron and first appeared in the USA in the 1970s [164]. The technique involves using a low-intensity electrical current to stimulate the cerebral cortex by application of electrodes to the patient’s earlobes (in a few reports, electrodes were attached to the mastoid processes and forehead) [134]. The current intensity is in the range of 50 μA to 4 mA [164], commonly under 2 mA (as in techniques such as tDCS and tRNS [165].
CES demonstrated satisfactory results as a treatment for depression, insomnia, post-traumatic stress disorder, and pain [164]. Moreover, the effectiveness of CES in anxiety treatment has been proven successful [165, 166]. Furthermore, this method also can be used in the pretreatment of preoperative anxiety [167].
The mechanisms underlying CES effects are currently unknown [164, 168]. CES effectiveness is suggested to be the result of the modulation of brain networks among the hypothalamus, limbic system, and reticular activating system. Moreover, imaging studies suggest that stimulation of the motor complex can result in modulating networks responsible for pain processing (thalamus), facilitating pain inhibitory mechanisms, and consequently reducing pain [134]. CES usage induces significant changes in a patient’s EEG. Alpha activity increases during stimulation, which correlates with relaxation, while beta and delta activity is decreased, which indicates a reduction in anxiety, ruminative thoughts, and fatigue [169]. Moreover, there is a supposed correlation between mechanisms of anxiety and chronic pain, having some data confirmation [165]. In this case, treatment aimed at an anxiety-causing mechanism could improve chronic pain as well. In patients experiencing chronic anxiety, enhanced chronic pain is often reported. Similarly, high preoperative anxiety levels can relate to increased pain sensing after surgery and more painful recovery. Indeed, it has been proven that anxiolytic treatments (medications and procedures) are beneficial in managing chronic pain [170]. These clinical observations were confirmed by human brain imaging studies, especially showing the role of the ACC, which is activated during anticipation of pain and influences anxiety perception (there is also a role of the amygdala and insular cortex) [170]. The mechanism that CES is believed to reduce anxiety levels may result from increased release of serotonin, endorphins, melatonin, and the concentration of γ-aminobutyric acid after stimulation [165, 169]. Therefore, this may be one of the mechanisms that CES is believed to improve chronic pain.
Another theoretical explanation for the mechanism of CES emphasizes the role of anti-nociceptive system activation, increasing serotonin, endorphins, and noradrenaline levels [171]. It has been observed that endorphin levels, decreased in the cerebrospinal fluid in patients with chronic pain, were rapidly increased after CES [171]. Moreover, the presence of endorphins in anti-nociceptive structures of the brain also confirms that hypothesis.
There exist few recent studies investigating the impact of CES on chronic pain. Older studies seemed to have proven benefits of this type of stimulation, but recent meta-analyses are not that optimistic. It has to be mentioned that there is a lack of large RCTs that could verify CES use in chronic pain treatment. Among available trials (Table 5), there is often a high risk of bias, and therefore the quality of evidence is low, which was emphasized by mentioned meta-analyses.
Besides some RCTs included in this paper, there are also systematic reviews worth mentioning, as the number of adequate RCTs is low and seems to need completing. In a systematic review by Shekelle, according to the analysis, there were again a small number of trials, and the risk of bias was high. The results evaluating the effectiveness of CES in chronic pain conditions were diverging. Some trials taken into analysis showed no statistically significant difference in pain scores compared to sham groups. Contrarily, other studies reported improvement in patients with such conditions as fibromyalgia, chronic neuromuscular pain, and musculoskeletal pain. Due to the high risk of bias, current evidence for CES effectiveness is considered insufficient [175]. Another study by this author presented similar conclusions [176].
Another systematic review found no improvement in chronic pain after using CES. This study aimed to determine the benefits of non-invasive brain stimulation methods in neuropathic pain after spinal cord injury. However, CES did not demonstrate any effects on pain and depression in examined patients [165].
A randomized controlled pilot study from 2020 examining the efficacy of CES in adults with osteoarthritis showed a positive impact on decreasing chronic pain. Active CES contributed to the reduction of scores on the Numeric Rating Scale. This randomized, double-blind, sham-controlled clinical trial featured remotely supervised CES, for 60 min daily, over 2 weeks (Monday to Friday), after previous proper instruction. The CES electrodes were attached to the patient’s earlobes. No adverse effects were reported during the trial. This study also figured out the difference in the functionality of the frontal cortex during active CES versus sham and under pain stimuli. This could suggest another mechanism of CES in reducing pain, as it induces a decrease in oxygenated hemoglobin in the frontal cortex. After all, CES in this study has been proven effective in managing chronic pain in OA. Moreover, it can be used remotely [172].
A study aiming to investigate the effects of CES therapy on symptoms in fibromyalgia showed a higher decrease in pain, fatigue, and sleep disturbance in the active CES treatment group, compared to the patients receiving either sham or usual care [173]. Therefore, the functional status of the active CES device group has increased. The study also investigated the effects of CES on both systolic and diastolic blood pressure, demonstrating no influence on these parameters and thus showing the safety of such therapy.
CES may also be beneficial for patients with advanced cancer, according to the results of a preliminary study [168]. The study has found that using CES in advanced cancer is safe for the patients and improves pain associated with the disease. After 4 weeks of treatment, there was a significant improvement in pain severity. Although these findings may seem promising, there were limitations in the study. The important one was the lack of a sham group, resulting in low reliability and a need for further well-designed research.
According to the current evidence, there have been no serious adverse events after CES using treatment. Adverse events such as pulsing, tingling, and tickling in the ears; tender ears; pins; and needles feeling near the bladder were reported in the group of actively stimulated patients versus drowsiness, warm ears, and headache after one session in the sham group (rare events, only in one participant each) in one study [177]. Also, one other study included information about adverse events, though these were mild such as sensations of ear pulsing, stinging, itching, electric sensations, or ear clip tightness [134, 178]. In general, these are the main adverse events we can expect.
In one early study, some patients experienced worsening depression after active CES, but it was probably related to the old type of used devices, no longer in use [179]. Other observed adverse effects were tiredness, malaise, sleepiness, skin irritation, and possible transient visual symptoms [176, 180], although another study revealed no reported adverse events during CES treatment [171].
Reduced impedance non-invasive cortical electrostimulation
RINCE is a much less described method of electrostimulation than previous ones. The current in this technique is applied by electrodes attached to the patient’s scalp. The main difference from the other stimulation techniques is that it uses specific current frequencies that allow deeper cortical penetration by reducing the impedance of the skin and skull. This enables low-frequency cortical modulation and is believed to increase signal transmission and stimulation effectiveness [134].
The effectiveness of RINCE in reducing pain was mentioned in the meta-analysis by O’Connell et al. It included only two articles regarding RINCE in chronic pain due to the lack of existing literature and studies (Table 5). In one of these studies, primarily unpublished (Deering 2017), the short-term follow-up demonstrated a positive impact on pain intensity. However, the rank of proof was poor. An improvement in quality of life was not observed [134]. Moreover, none of the included studies have shown any superiority of stimulation over sham. The study that we could reach [174] was a RCT investigating RINCE in managing symptoms of fibromyalgia patients. The results suggest a positive impact of active RINCE therapy on managing fibromyalgia symptoms. During treatment, both mean tender points and pressure pain threshold represented an improvement in the active group compared to the sham group. There was also a decrease in pain VAS scores in patients receiving active RINCE treatment. The outcomes were statistically and clinically significant, suggesting the advantages of using a RINCE device in such conditions.
Adverse events after RINCE include headache (mild to moderate intensity), nausea, dizziness, vertigo, and localized skin reactions. These side effects were mild to moderate and occurred averagely with a frequency of two complications per patient, but fortunately were short-lived and disappeared with no intervention. Other events reported by another included study were eye movement and restlessness, with low incidence [134].
Conclusion
In recent decades, numerous researchers extensively debated the clinical usefulness of brain stimulation as a treatment for chronic pain.
The exact phenomena underlying the analgesic mechanisms of brain stimulation are still unrecognized in the case of all techniques discussed in this review. However, various hypotheses on their probable mechanism of action have been formed in recent decades, and evidence in this field constantly grows. The enhancement of knowledge about pain relief mechanisms by brain stimulation may significantly contribute to improving the current clinical outcomes of this approach.
The majority of studies conducted to date on the use of brain stimulation in chronic pain are burdened with small sample sizes and poor scientific methodology.
DBS stands out from the rest of the methods in its efficacy in the treatment of cluster headaches. Regarding MCS, there is inconsistency as regards the results of published to-date clinical studies. The identification of specific subgroups of patients well responding to MCS is still under investigation. Although due to the high complication rate of invasive brain stimulation methods, their use should be appropriate only when the chronic pain is refractory to other available therapeutic methods.
Since tDCS and rTMS demonstrated successful results on chronic pain relief with a low rate of side effects, their clinical use is the most beneficial among discussed techniques. Moreover, current research on adverse effects prevention is continued to further increase the safety of these methods.
Regarding CES and RINCE, it is clear that there is a need for further research to confirm findings and their reliability because the quality of evidence remains very low. There are only a few studies that suggest CES therapy to be beneficial and most clinical trials showed no benefit. RINCE was proven effective in managing chronic pain, but data referred only to one study, so there was the risk of bias due to the small sample size.
In summary, rTMS and tDCS represent the most promising therapeutic options for chronic pain among discussed brain stimulation methods. However, due to the low quality of evidence provided by available studies, large multi-center RCTs with long-term follow-ups are necessary to verify the safety and clinical outcomes of non-invasive as well as invasive brain stimulation.
References
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von KM, Porter L, Helmick C (2018) Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. Morb Mortal Wkly Rep. 67(36):1001–1006. https://doi.org/10.15585/mmwr.mm6736a2
Yong RJ, Mullins PM, Bhattacharyya N (2022) Prevalence of chronic pain among adults in the United States. Pain. 163(2):E328–E332
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D (2006) Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 10(4):287–287
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH et al (2015) A classification of chronic pain for ICD-11. Pain. 156(6):1003–1007
Cohen SP, Vase L, Hooten WM (2021) Chronic pain: an update on burden, best practices, and new advances. Lancet (London, England). 397(10289):2082–2097
Mills SEE, Nicolson KP, Smith BH (2019) Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 123(2):e273–e283
Relieving Pain in America (2011) a blueprint for transforming prevention, care, education, and research. Reli Pain Am A Bluepr Transform Prev Care, Educ Res. 26:1–364
Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, Cohen M, Evers S, Giamberardino MA, Goebel A, Korwisi B, Perrot S, Svensson P, Wang SJ, Treede RD (2019) The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 160(1):28–37
Zhang H (2022) Cancer pain management-new therapies. Curr Oncol Rep. 24(2):223–226
Litak J, Czyżewski W, Szymoniuk M, Sakwa L, Pasierb B, Litak J, Hoffman Z, Kamieniak P, Roliński J (2022) Biological and clinical aspects of metastatic spinal tumors. Cancers (Basel). 14(19):4599
Sluka KA, Clauw DJ (2016) Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 3(338):114–129
Szewczyk AK, Jamroz-Wiśniewska A, Rejdak K (2023) Possible neuropathic pain in clinical practice - review on selected diagnostic tools and its further challenges. Diagnostics. 13(1):108
Bielewicz J, Kamieniak M, Szymoniuk M, Litak J, Czyżewski W, Kamieniak P (2023) Diagnosis and management of neuropathic pain in spine diseases. J Clin Med. 12:4
Wylde V, Dennis J, Beswick AD, Bruce J, Eccleston C, Howells N, Peters TJ, Gooberman-Hill R (2017) Systematic review of management of chronic pain after surgery. Br J Surg. 104(10):1293–1306
Attal N (2021) Spinal cord injury pain. Rev Neurol (Paris). 177(5):606–612
Ventafridda V, Saita L, Ripamonti C, De Conno F (1985) WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 7(1):93–96
George B, Minello C, Allano G, Maindet C, Burnod A, Lemaire A (2019) Opioids in cancer-related pain: current situation and outlook. Supp Care Cancer. 27(8):3105–3118
Kalso E, Edwards JE, Moore RA, McQuay HJ (2004) Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 112(3):372–380
Els C, Jackson TD, Kunyk D, Lappi VG, Sonnenberg B, Hagtvedt R, Sharma S, Kolahdooz F, Straube S (2017) Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: an overview of Cochrane Reviews. Cochrane database Syst Rev. 10:10
Avery N, McNeilage AG, Stanaway F, Ashton-James CE, Blyth FM, Martin R, Gholamrezaei A, Glare P (2022) Efficacy of interventions to reduce long term opioid treatment for chronic non-cancer pain: systematic review and meta-analysis. BMJ. 4:377
Katta MR, Valisekka SS, Agarwal P, Hameed M, Shivam S, Kaur J, Prasad S, Bethineedi LD, Lavu DV, Katamreddy Y (2022) Non-pharmacological integrative therapies for chronic cancer pain. J Oncol Pharm Pract. 28(8):1859–1868
Kaye AD, Cornett EM, Hart B, Patil S, Pham A, Spalitta M, Mancuso KF (2018) Novel Pharmacological nonopioid therapies in chronic pain. Curr Pain Headache Rep. 22:4
Urits I, Gress K, Charipova K, Habib K, Lee D, Lee C, Jung JW, Kassem H, Cornett E, Paladini A, Varrassi G, Kaye AD, Viswanath O (2020) Use of cannabidiol (CBD) for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 34(3):463–477
Deer TR, Jain S, Hunter C, Chakravarthy K (2019) Neurostimulation for intractable chronic pain. Brain Sci. 9:2
Ren J, Liu N, Sun N, Zhang K, Yu L (2019) Mesenchymal stem cells and their exosomes: promising therapeutics for chronic pain. Curr Stem Cell Res Ther. 14(8):644–653
Szymoniuk M, Litak J, Sakwa L, Dryla A, Zezuliński W, Czyżewski W, Kamieniak P, Blicharski T (2022) Molecular mechanisms and clinical application of multipotent stem cells for spinal cord injury. Cells. 1:120
Li J, Szabova A (2021) Ultrasound-guided nerve blocks in the head and neck for chronic pain management: the anatomy, sonoanatomy, and procedure. Pain Physician. 24(8):533–548
Dale R, Stacey B (2016) Multimodal treatment of chronic pain. Med Clin North Am. 100(1):55–64
Hylands-White N, Duarte RV, Raphael JH (2017) An overview of treatment approaches for chronic pain management. Rheumatol Int. 37(1):29–42
Meints SM, Edwards RR (2018) Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry. 87(Part B):168–182
Bicego A, Monseur J, Collinet A, Donneau AF, Fontaine R, Libbrecht D, Malaise N, Nyssen AS, Raaf M, Rousseaux F, Salamun I, Staquet C, Teuwis S, Tomasella M, Faymonville ME, Vanhaudenhuyse A (2021) Complementary treatment comparison for chronic pain management: A randomized longitudinal study. PLoS One. 16:8
Lanska DJ (2002) Corning and vagal nerve stimulation for seizures in the 1880s. Neurology. 58(3):452–459
Kogan M, McGuire M, Riley J (2019) Deep brain stimulation for Parkinson disease. Neurosurg Clin N Am. 30(2):137–146
Schuepbach WMM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Hälbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH et al (2013) Neurostimulation for Parkinson’s disease with early motor complications. N Engl J Med. 368(7):610–622
Gruber D, Südmeyer M, Deuschl G, Falk D, Krauss JK, Mueller J, Müller JU, Poewe W, Schneider GH, Schrader C, Vesper J, Volkmann J, Winter C, Kupsch A, Schnitzler A (2018) Neurostimulation in tardive dystonia/dyskinesia: a delayed start, sham stimulation-controlled randomized trial. Brain Stimul. 6:1368–1377
Sobstyl M, Stapińska-Syniec A, Rylski M (2020) Deep brain stimulation for the treatment of refractory and super-refractory status epilepticus. Seizure. 1(81):58–62
Helm S, Shirsat N, Calodney A, Abd-Elsayed A, Kloth D, Soin A, Shah S, Trescot A (2021) Peripheral nerve stimulation for chronic pain: a systematic review of effectiveness and safety. Pain Ther. 10(2):985–1002
Grider J, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, Grami V, Justiz R, Nouri K, Hayek SM, Vallejo R, Christo PJ (2016) Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician. 19(1):E33–E54
Alamri A, Pereira EAC (2022) Deep brain stimulation for chronic pain. Neurosurg Clin N Am. 33(3):311–321
Xiong HY, Zheng JJ, Wang XQ (2022 May) Non-invasive brain stimulation for chronic pain: state of the art and future directions. Front Mol Neurosci. 26:15
Nevro Corp. - Nevro Announces FDA Approval of its 10 kHz high frequency spinal cord stimulation therapy for treatment of chronic pain associated with painful diabetic neuropathy (PDN) [Internet]. [cited 2023 Jan 9]. Available from: https://nevro.com/English/us/investors/investor-news/investor-news-details/2021/Nevro-Announces-FDA-Approval-of-its-10-kHz-High-Frequency-Spinal-Cord-Stimulation-Therapy-for-Treatment-of-Chronic-Pain-Associated-with-Painful-Diabetic-Neuropathy-PDN/default.aspx
Medtronic announces FDA approval of spinal cord stimulation therapy for treating chronic pain resulting from diabetic peripheral neuropathy - Jan 24, 2022 [Internet]. [cited 2023 Jan 9]. Available from: https://news.medtronic.com/2022-01-24-Medtronic-announces-FDA-approval-of-spinal-cord-stimulation-therapy-for-treating-chronic-pain-resulting-from-diabetic-peripheral-neuropathy
Baethge C, Goldbeck-Wood S, Mertens S (2019) SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 4(1):1–7
Knotkova H, Hamani C, Sivanesan E, Le Beuffe MFE, Moon JY, Cohen SP, Huntoon MA (2021) Neuromodulation for chronic pain. Lancet. 397(10289):2111–2124
Abreu V, Vaz R, Chamadoira C, Rebelo V, Reis C, Costa F, Martins J, Gillies MJ, Aziz TZ, Pereira EAC (2022) Thalamic deep brain stimulation for post-traumatic neuropathic limb pain: efficacy at five years’ follow-up and effective volume of activated brain tissue. Neurochirurgie. 68(1):52–60
Fontaine D, Lazorthes Y, Mertens P, Blond S, Géraud G, Fabre N, Navez M, Lucas C, Dubois F, Gonfrier S, Paquis P, Lantéri-Minet M (2010) Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 11(1):23–31
Moisset X, Lanteri-Minet M, Fontaine D (2019) Neurostimulation methods in the treatment of chronic pain. J Neural Transm. 127(4):673–686
Schoenen J, Jensen RH, Lantéri-Minet M, Láinez MJA, Gaul C, Goodman AM, Caparso A, May A (2013) Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 33(10):816–830
Sims-Williams H, Matthews JC, Talbot PS, Love-Jones S, Brooks JC, Patel NK, Pickering AE (2017) Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. Neuroimage. 2(146):833
Lubejko ST, Graham RD, Livrizzi G, Schaefer R, Banghart MR, Creed MC (2022) The role of endogenous opioid neuropeptides in neurostimulation-driven analgesia. Front Syst Neurosci. 14(16):132
Bergeron D, Obaid S, Fournier-Gosselin MP, Bouthillier A, Nguyen DK (2021) Deep brain stimulation of the posterior insula in chronic pain: a theoretical framework. Brain Sci. 11(5):639
Shirvalkar P, Veuthey TL, Dawes HE, Chang EF (2018) Closed-loop deep brain stimulation for refractory chronic pain. Front Comput Neurosci. 26(12):18
Gopalakrishnan R, Burgess RC, Malone DA, Lempka SF, Gale JT, Floden DP, Baker KB, Machado AG (2018) Deep brain stimulation of the ventral striatal area for poststroke pain syndrome: a magnetoencephalography study. J Neurophysiol. 119(6):2118–2128
Lempka SF, Malone DA, Hu B, Baker KB, Wyant A, Ozinga JG, Plow EB, Pandya M, Kubu CS, Ford PJ, Machado AG (2017) Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 81(5):653–663
Belasen A, Rizvi K, Gee LE, Yeung P, Prusik J, Ramirez-Zamora A, Hanspal E, Paiva P, Durphy J, Argoff CE, Pilitsis JG (2017) Effect of low-frequency deep brain stimulation on sensory thresholds in Parkinson’s disease. J Neurosurg. 126(2):397–403
Son BC, Kim DR, Kim HS, Lee SW (2014) Simultaneous trial of deep brain and motor cortex stimulation in chronic intractable neuropathic pain. Stereotact Funct Neurosurg. 92(4):218–226
Marques A, Chassin O, Morand D, Pereira B, Debilly B, Derost P, Ulla M, Lemaire JJ, Durif F (2013) Central pain modulation after subthalamic nucleus stimulation: a crossover randomized trial. Neurology. 81(7):633–640
Lenoir C, Algoet M, Mouraux A (2018) Deep continuous theta burst stimulation of the operculo-insular cortex selectively affects Aδ-fibre heat pain. J Physiol. 596(19):4767–4787
Sanger TD (2020) Deep brain stimulation for cerebral palsy: where are we now? Dev Med Child Neurol. 62(1):28–33
Ostergard T, Munyon C, Miller JP (2014) Motor cortex stimulation for chronic pain. Neurosurg Clin N Am. 25(4):693–698
Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S (1991) Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 14(1):131–134
Ramos-Fresnedo A, Perez-Vega C, Domingo RA, Cheshire WP, Middlebrooks EH, Grewal SS (2022) Motor cortex stimulation for pain: a narrative review of indications, techniques, and outcomes. Neuromodulation Technol Neural Interface. 25(2):211–221
Tronnier V, Rasche D (2013) Epidural and subdural stimulation. Handb Clin Neurol. 116:343–351
Zhang X, Hu Y, Tao W, Zhu H, Xiao D, Li Y (2017) The effect of motor cortex stimulation on central poststroke pain in a series of 16 patients with a mean follow-up of 28 months. Neuromodulation Technol Neural Interface. 20(5):492–496
Buchanan RJ, Darrow D, Monsivais D, Nadasdy Z, Gjini K (2014) Motor cortex stimulation for neuropathic pain syndromes: a case series experience. Neuroreport. 25(9):721–723
Lopes PSS, Campos ACP, Fonoff ET, Britto LRG, Pagano RL (2019) Motor cortex and pain control: exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav Brain Funct. 15:1
Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LRG (2012) Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: Possible pathways for antinociception. PAIN®. 153:2359–2369
Zhao R, Zhou H, Huang L, Xie Z, Wang J, Gan WB, Yang G (2018) Neuropathic pain causes pyramidal neuronal hyperactivity in the anterior cingulate cortex. Front Cell Neurosci. 20(12):107
Cha M, Um SW, Kwon M, Nam TS, Lee BH (2017) Repetitive motor cortex stimulation reinforces the pain modulation circuits of peripheral neuropathic pain. Sci Rep. 7:1
Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L (2007) Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 69(9):827–834
Silva GD, Lopes PSS, Fonoff ET, Pagano RL (2015) The spinal anti-inflammatory mechanism of motor cortex stimulation: cause of success and refractoriness in neuropathic pain? J Neuroinflammation. 12:1
Fontaine D, Hamani C, Lozano A (2009) Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature - Clinical article. J Neurosurg. 110(2):251–256
Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco BA, Navarro J, Yeng LT, Teixeira MJ, De Andrade DC (2021) Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain. 144(10):2994–3004
Sokal P, Harat M, Malukiewicz A, Kiec M, Świtońska M, Jabłońska R (2019) Effectiveness of tonic and burst motor cortex stimulation in chronic neuropathic pain. J Pain Res. 12:1863
Henssen DJ, Kurt E, Cappellen van Walsum AM, Arnts I, Doorduin J, Kozicz T, Van Dongen R, Bartels RH (2018) Long-term effect of motor cortex stimulation in patients suffering from chronic neuropathic pain: an observational study. PLoS One. 13:1
Tanei T, Kajita Y, Maesawa S, Nakatsubo D, Aoki K, Noda H, Takebayashi S, Nakahara N, Wakabayashi T (2018) Long-term effect and predictive factors of motor cortex and spinal cord stimulation for chronic neuropathic pain. Neurol Med Chir (Tokyo). 58(10):422
Zhang X, Zhu H, Tao W, Li Y, Hu Y (2018) Motor cortex stimulation therapy for relief of central post-stroke pain: a retrospective study with neuropathic pain symptom inventory. Stereotact Funct Neurosurg. 96(4):239–243
Ivanishvili Z, Poologaindran A, Honey CR (2017) Cyclization of motor cortex stimulation for neuropathic pain: a prospective, randomized, blinded trial. Neuromodulation Technol Neural Interface. 20(5):497–503
Rasche D, Tronnier VM (2016) Clinical significance of invasive motor cortex stimulation for trigeminal facial neuropathic pain syndromes. Neurosurgery. 79(5):655–665
Kolodziej MA, Hellwig D, Nimsky C, Benes L (2015) Treatment of central deafferentation and trigeminal neuropathic pain by motor cortex stimulation: report of a series of 20 patients. J Neurol Surgery, Part A Cent Eur Neurosurg. 77(1):52–58
Radic JAE, Beauprie I, Chiasson P, Kiss ZHT, Brownstone RM (2015) Motor cortex stimulation for neuropathic pain: a randomized cross-over trial. Can J Neurol Sci. 42(6):401–409
Sokal P, Harat M, Zieliński P, Furtak J, Paczkowski D, Rusinek M (2015) Motor cortex stimulation in patients with chronic central pain. Adv Clin Exp Med. 24(2):289–296
Isagulyan ED, Tomsky AA, Dekopov AV, Salova EM, Troshina EM, Dorokhov EV, Shabalov VA (2015) Results of motor cortex stimulation in the treatment of chronic pain syndromes. Zh Vopr Neirokhir Im N N Burdenko. 79(6):46–60
Im SH, Ha SW, Kim DR, Son BC (2015, May28) Long-Term Results of Motor Cortex Stimulation in the Treatment of Chronic, Intractable Neuropathic Pain. Stereotact Funct Neurosurg. 93(3):212–8
André-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L (2014) Is Life better after motor cortex stimulation for pain control? Results at long-term and their prediction by preoperative rTMS. Pain Physician. 17(1):53–62
Sachs AJ, Babu H, Su YF, Miller KJ, Henderson JM (2014, Jun 1) Lack of Efficacy of Motor Cortex Stimulation for the Treatment of Neuropathic Pain in 14 Patients. Neuromodulation Technol Neural Interface. 17(4):303–11
Delavallée M, Finet P, de Tourtchaninoff M, Raftopoulos C (2014) Subdural motor cortex stimulation: feasibility, efficacy and security on a series of 18 consecutive cases with a follow-up of at least 3 years. Acta Neurochir (Wien). 156(12):2289–2294
Parravano DC, Ciampi DA, Fonoff ET, Monaco B, Navarro J, Yeng LT, Teixeira MJ, Hamani C (2019) Quality of life after motor cortex stimulation: clinical results and systematic review of the literature. Neurosurgery. 84(2):451–456
Henssen D, Kurt E, van Walsum AMVC, Kozicz T, van Dongen R, Bartels R (2020) Motor cortex stimulation in chronic neuropathic orofacial pain syndromes: a systematic review and meta-analysis. Sci Rep. 10:1
Antony AB, Mazzola AJ, Dhaliwal GS, Hunter CW (2019) Neurostimulation for the treatment of chronic head and facial pain: a literature review. Pain Physician. 22(5):447–477
Kustermans L, Van Buyten JP, Smet I, Coucke W, Politis C (2017) Stimulation of the Gasserian ganglion in the treatment of refractory trigeminal neuropathy. J Craniomaxillofac Surg. 45(1):39–46
Klein J, Siepmann T, Schackert G, Ziemssen T, Juratli TA (2020) Peripheral nerve field stimulation in medically refractory trigeminal neuralgia attributed to multiple sclerosis. J Neurosurg. 134(3):1244–1250
Rapisarda A, Ioannoni E, Izzo A, Montano N (2021) What are the results and the prognostic factors of motor cortex stimulation in patients with facial pain? A Systematic Review of the Literature. Eur Neurol. 84(3):151–156
Thair H, Holloway AL, Newport R, Smith AD (2017) Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci. 11:641
Dondé C, Amad A, Nieto I, Brunoni AR, Neufeld NH, Bellivier F, Poulet E, Geoffroy PA (2017) Transcranial direct-current stimulation (tDCS) for bipolar depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 1(78):123–131
Brunoni AR, Moffa AH, Sampaio-Júnior B, Gálvez V, Loo CK (2017) Treatment-emergent mania/hypomania during antidepressant treatment with transcranial direct current stimulation (tDCS): a systematic review and meta-analysis. Brain Stimul. 10(2):260–262
Freire RC, Cabrera-Abreu C, Milev R (2020) Neurostimulation in anxiety disorders, post-traumatic stress disorder, and obsessive-compulsive disorder. Adv Exp Med Biol. 1191:331–346
Jiang WL, Bin CD, Sun CH, Yin F, Goerigk S, Brunoni AR, Zhao XW, Mayes TL, Zheng W, Xiang YT (2022) Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: a meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J Psychiatr. 73:103100
Saxena V, Pal A (2021) Role of transcranial direct current stimulation in the management of Alzheimer’s disease: a meta-analysis of effects, adherence and adverse effects. Clin Psychopharmacol Neurosci. 19(4):589–599
Bai Y, Xia X, Kang J, Yang Y, He J, Li X (2017) TDCS modulates cortical excitability in patients with disorders of consciousness. Neuroimage (Amst). 15:702
Yokoi Y, Narita Z, Sumiyoshi T (2017) Transcranial direct current stimulation in depression and psychosis: a systematic review. Clin EEG Neurosci. 49:93–102
Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 127(2):1031–1048
van Dun K, Bodranghien FCAA, Mariën P, Manto MU (2016) TDCS of the cerebellum: where do we stand in 2016? Technical issues and critical review of the literature. Front Hum Neurosci. 10(MAY2016):199
Ahn H, Woods AJ, Kunik ME, Bhattacharjee A, Chen Z, Choi E, Fillingim RB (2017) Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: an experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul. 10(5):902
De Souza CG, Pegado R, Costa J, Morya E, Baptista AF, Unal G, Bikson M, Okano AH (2021) Alternate sessions of transcranial direct current stimulation (tDCS) reduce chronic pain in women affected by chikungunya. A randomized clinical trial. Brain Stimul. 14(3):541–548
Khedr EM, Omran EAH, Ismail NM, El-Hammady DH, Goma SH, Kotb H, Galal H, Osman AM, Farghaly HSM, Karim AA, Ahmed GA (2017) Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimul. 10(5):893–901
Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, Fregni F (2013) Focal modulation of the primary motor cortex in fibromyalgia using 4×1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. J pain. 14(4):371–383
Ayache SS, Palm U, Chalah MA, Al-Ani T, Brigno A, Abdellaoui M, Dimitri D, Sorel M, Créange A, Lefaucheur JP (2016) Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci. 10(APR):147
Luedtke K, Rushton A, Wright C, Jürgens T, Polzer A, Mueller G, May A (2015) Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. BMJ. 16:350
O’Neill F, Sacco P, Bowden E, Asher R, Burnside G, Cox T, Nurmikko T (2018) Patient-delivered tDCS on chronic neuropathic pain in prior responders to TMS (a randomized controlled pilot study). J Pain Res. 11:3117–3128
Fregni F, Macedo IC, Spezia-Adachi LN, Scarabelot VL, Laste G, Souza A, Sanches PRS, Caumo W, Torres ILS (2018) Transcranial direct current stimulation (tDCS) prevents chronic stress-induced hyperalgesia in rats. Brain Stimul. 11(2):299–301
Chang WJ, Bennell KL, Hodges PW, Hinman RS, Young CL, Buscemi V, Liston MB, Schabrun SM (2017) Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: a pilot randomised controlled trial. PLoS One. 12:6
Harvey MP, Lorrain D, Martel M, Bergeron-Vezina K, Houde F, Séguin M, Léonard G (2017) Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? – Results from a randomized controlled pilot study. Clin Interv Aging. 6(12):937
Hazime FA, Baptista AF, de Freitas DG, Monteiro RL, Maretto RL, Hasue RH, João SMA (2017) Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double-blind, factorial clinical trial. Eur J Pain. 21(7):1132–1143
Lagueux E, Bernier M, Bourgault P, Whittingstall K, Mercier C, Léonard G, Laroche S, Tousignant-Laflamme Y (2018) The effectiveness of transcranial direct current stimulation as an add-on modality to graded motor imagery for treatment of complex regional pain syndrome: a randomized proof of concept study. Clin J Pain. 34(2):145–154
Thibaut A, Carvalho S, Morse LR, Zafonte R, Fregni F (2017) Delayed pain decrease following M1 tDCS in spinal cord injury: a randomized controlled clinical trial. Neurosci Lett. 29(658):19–26
Brietzke AP, Rozisky JR, Dussan-Sarria JA, Deitos A, Laste G, Hoppe PFT, Muller S, Torres ILS, Alvares-da-Silva MR, de Amorim RFB, Fregni F, Caumo W (2015) Neuroplastic effects of transcranial direct current stimulation on painful symptoms reduction in chronic hepatitis C: a phase II randomized, double blind, sham controlled trial. Front Neurosci. 9(JAN):498
Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F (2016) Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo-controlled clinical trial. Front Hum Neurosci. 10(MAR2016):68
Volz MS, Farmer A, Siegmund B (2016) Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. Pain. 157(2):429–437
Donnell A, Nascimento TD, Lawrence M, Gupta V, Zieba T, Truong DQ, Bikson M, Datta A, Bellile E, DaSilva AF (2015) High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimul. 8(6):1085
Ngernyam N, Jensen MP, Arayawichanon P, Auvichayapat N, Tiamkao S, Janjarasjitt S, Punjaruk W, Amatachaya A, Aree-uea B, Auvichayapat P (2015) The effects of transcranial direct current stimulation in patients with neuropathic pain from spinal cord injury. Clin Neurophysiol. 126(2):382–390
Sakrajai P, Janyacharoen T, Jensen MP, Sawanyawisuth K, Auvichayapat N, Tunkamnerdthai O, Keeratitanont K, Auvichayapat P (2014) Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clin J Pain. 30(12):1076
Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, Diener HC, Obermann M (2014) Patient-conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. J Headache Pain. 15(1):78
Souto G, Borges IC, Goes BT, De Mendonça ME, Gonçalves RG, Garcia LB, Sá KN, Coutinho MR, Galvão-Castro B, Fregni F, Baptista AF (2014) Effects of tDCS-induced motor cortex modulation on pain in HTLV-1: a blind randomized clinical trial. Clin J Pain. 30(9):809–815
Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ (2013) Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain. 154(10):2178–2184
Jensen MP, Sherlin LH, Askew RL, Fregni F, Witkop G, Gianas A, Howe JD, Hakimian S (2013, Oct) Effects of non-pharmacological pain treatments on brain states. Clin Neurophysiol. 124(10):2016
Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, Kang YJ (2013) Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Ann Rehabil Med. 37(6):766
Fregni F, Boggio PS, Lima MC, Ferreira MJL, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A (2006) A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 122(1–2):197–209
Fregni F, Gimenes R, Valle AC, Ferreira MJL, Rocha RR, Natalle L, Bravo R, Rigonatti SP, Freedman SD, Nitsche MA, Pascual-Leone A, Boggio PS (2006) A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 54:3988–3998
Boggio PS, Amancio EJ, Correa CF, Cecilio S, Valasek C, Bajwa Z, Freedman SD, Pascual-Leone A, Edwards DJ, Fregni F (2009) Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clin J Pain. 25(8):691–695
Mori F, Codecà C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, Bernardi G, Koch G, Centonze D (2010) Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J pain. 11(5):436–442
Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, Navarro X, Pascual-Leone A (2010) Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 133(9):2565–2577
Navarro-López V, Del-Valle-Gratacós M, Fernández-Vázquez D, Fernández-González P, Carratalá-Tejada M, Molina-Rueda F (2022) Transcranial direct current stimulation in the management of phantom limb pain: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 58(5):738–748
O’Connell NE, Marston L, Spencer S, DeSouza LH, Wand BM (2018) Non-invasive brain stimulation techniques for chronic pain. Cochrane database Syst Rev. 4:4
Poreisz C, Boros K, Antal A, Paulus W (2007) Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 72(4–6):208–214
Ma Q, Pu M, Li M, Haihambo N, Baetens K, Heleven E, Deroost N, Baeken C, Van Overwalle F (2023) Can transcranial direct current stimulation (tDCS) of the cerebellum improve implicit social and cognitive sequence learning? Int J Clin Health Psychol. 23(2):100355
Martin DM, Alonzo A, Mitchell PB, Sachdev P, Gálvez V, Loo CK (2011) Fronto-extracephalic transcranial direct current stimulation as a treatment for major depression: an open-label pilot study. J Affect Disord. 134(1–3):459–463
Lippold OC, Redfearn JW (1964) Mental changes resulting from the passage of small direct currents through the human brain. Br J Psychiatry. 110(469):768–772
Sonmez AI, Camsari DD, Nandakumar AL, Voort JLV, Kung S, Lewis CP, Croarkin PE (2019) Accelerated TMS for depression: a systematic review and meta-analysis. Psychiatry Res. 1(273):770–781
Sabbagh M, Sadowsky C, Tousi B, Agronin ME, Alva G, Armon C, Bernick C, Keegan AP, Karantzoulis S, Baror E, Ploznik M, Pascual-Leone A (2020) Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimers Dement. 16(4):641–650
Chung CLH, Mak MKY, Hallett M (2020) Transcranial magnetic stimulation promotes gait training in Parkinson disease. Ann Neurol. 88(5):933–945
Uzair M, Abualait T, Arshad M, Yoo WK, Mir A, Bunyan R, Bashir S (2022) Transcranial magnetic stimulation in animal models of neurodegeneration. Neural Regen Res. 17(2):251
Galletta EE, Rao PR, Barrett AM (2011) Transcranial magnetic stimulation (TMS): potential progress for language improvement in aphasia. Top Stroke Rehabil. 18(2):87
Doyal A, Schoenherr JW, Flynn DN (2022) Motor evoked potential. StatPearls
Ojala J, Vanhanen J, Harno H, Lioumis P, Vaalto S, Kaunisto MA, Putaala J, Kangasniemi M, Kirveskari E, Mäkelä JP, Kalso E (2022) A randomized, sham-controlled trial of repetitive transcranial magnetic stimulation targeting M1 and S2 in central poststroke pain: a pilot trial. Neuromodulation. 25(4):538–548
De Risio L, Borgi M, Pettorruso M, Miuli A, Ottomana AM, Sociali A, Martinotti G, Nicolò G, Macrì S, di Giannantonio M, Zoratto F (2020) Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): a systematic review and meta-analysis of preclinical studies. Transl Psychiatry. 10:1
Ansari AH, Pal A, Ramamurthy A, Kabat M, Jain S, Kumar S (2021) Fibromyalgia pain and depression: an update on the role of repetitive transcranial magnetic stimulation. ACS Chem Neurosci. 12(2):256–270
Yang L, Wang SH, Hu Y, Sui YF, Peng T, Guo TC (2018) Effects of repetitive transcranial magnetic stimulation on astrocytes proliferation and nNOS expression in neuropathic pain rats. Curr. Med Sci. 38(3):482–490
Säisänen L, Huttunen J, Hyppönen J, Nissen M, Kotiranta U, Mervaala E, von Fraunberg M (2022) Efficacy and tolerability in patients with chronic facial pain of two consecutive treatment periods of rTMS applied over the facial motor cortex, using protocols differing in stimulation frequency, duration, and train pattern. Neurophysiol Clin. 52(2):95–108
Bursali C, Özkan FÜ, Kaysin MY, Dortcan N, Aktas I, Külcü DG (2021) Effectiveness of repetitive transcranial magnetic stimulation in patients with failed back surgery syndrome: a double-blind randomized placebo-controlled study. Pain Physician. 24(1):E23–E30
Kumar A, Mattoo B, Bhatia R, Kumaran S, Bhatia R (2021) Neuronavigation based 10 sessions of repetitive transcranial magnetic stimulation therapy in chronic migraine: an exploratory study. Neurol Sci. 42(1):131–139
Mattoo B, Tanwar S, Bhatia R, Tripathi M, Bhatia R (2019) Repetitive transcranial magnetic stimulation in chronic tension-type headache: a pilot study. Indian J Med Res. 150(1):73–80
Choi GS, Kwak SG, Do LH, Chang MC (2018) Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: a pilot study. J Rehabil Med. 50(3):246–252
Fitzgibbon BM, Hoy KE, Knox LA, Guymer EK, Littlejohn G, Elliot D, Wambeek LE, McQueen S, Elford KA, Lee SJ, Enticott PG, Fitzgerald PB (2018) Evidence for the improvement of fatigue in fibromyalgia: a 4-week left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation randomized-controlled trial. Eur J Pain. 22(7):1255–1267
Choi G, sik, Chang MC. (2018) Effects of high-frequency repetitive transcranial magnetic stimulation on reducing hemiplegic shoulder pain in patients with chronic stoke: a randomized controlled trial. Int J Neurosci. 128(2):110–116
Nurmikko T, MacIver K, Bresnahan R, Hird E, Nelson A, Sacco P (2016) Motor cortex reorganization and repetitive transcranial magnetic stimulation for pain-a methodological study. Neuromodulation. 19(7):669–678
Leung A, Shukla S, Fallah A, Song D, Lin L, Golshan S, Tsai A, Jak A, Polston G, Lee R (2016) Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation. 19(2):133–141
Umezaki Y, Badran BW, Devries WH, Moss J, Gonzales T, George MS (2016) The efficacy of daily prefrontal repetitive transcranial magnetic stimulation (rTMS) for burning mouth syndrome (BMS): a randomized controlled single-blind study. Brain Stimul. 9(2):234–242
Fricová J, Klírová M, Masopust V, Novák T, Vérebová K, Rokyta R (2013) Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain. Physiol Res. 62:1
Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipović SR, Hummel FC, Jääskeläinen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T et al (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 125(11):2150–2206
Moisset X, Bouhassira D, Attal N (2021) French guidelines for neuropathic pain: an update and commentary. Rev Neurol (Paris). 177(7):834–837
Quesada C, Pommier B, Fauchon C, Bradley C, Créac'h C, Murat M, Vassal F, Peyron R (2020) New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study. Pain. 161(4):718–728
Komatsu T, Hada T, Sasaki N, Kida H, Takahashi J, Maku T, Nakada R, Shiraishi T, Akiyama S, Kitagawa T, Sato T, Takatsu H, Sakuta K, Sakai K, Umehara T, Omoto S, Murakami H, Mitsumura H, Abo M, Iguchi Y (2022) Effects and safety of high-frequency rTMS in acute intracerebral hemorrhage patients: a pilot study. J Neurol Sci. 443:120473
Brunyé TT, Patterson JE, Wooten T, Hussey EK (2021 Feb) A critical review of cranial electrotherapy stimulation for neuromodulation in clinical and non-clinical samples. Front Hum Neurosci. 1(15):12
Yu B, Qiu H, Li J, Zhong C, Li J (2020) Noninvasive brain stimulation does not improve neuropathic pain in individuals with spinal cord injury: evidence from a meta-analysis of 11 randomized controlled trials. Am J Phys Med Rehabil. 99(9):811–820
Ching PY, Hsu TW, Chen GW, Pan CC, Chu CS, Chou PH (2022) Efficacy and tolerability of cranial electrotherapy stimulation in the treatment of anxiety: a systemic review and meta-analysis. Front psychiatry. 9:13
Kang HW, Kim HJ, Kim WY, Min WK, Min TJ, Lee YS, Kim JH (2020) Effects of cranial electrotherapy stimulation on preoperative anxiety and blood pressure during anesthetic induction in patients with essential hypertension. J Int Med Res. 48:8
Yennurajalingam S, Kang DH, Hwu WJ, Padhye NS, Masino C, Dibaj SS, Liu DD, Williams JL, Lu Z, Bruera E (2018) Cranial electrotherapy stimulation for the management of depression, anxiety, sleep disturbance, and pain in patients with advanced cancer: a preliminary study. J Pain Symptom Manage. 55(2):198–206
Kirsch DL, Nichols F (2013 Mar) Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 36(1):169–176
Zhuo M (2016) Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci. 39(3):136–145
Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D (2009) Pain reduction using transcranial electrostimulation: a double blind “active placebo” controlled trial. J Rehabil Med. 41(4):256–261
Ahn H, Galle K, Mathis KB, Miao H, Montero-Hernandez S, Jackson N, Ju HH, McCrackin H, Goodwin C, Hargraves A, Jain B, Dinh H, Abdul-Mooti S, Park L, Pollonini L (2020) Feasibility and efficacy of remotely supervised cranial electrical stimulation for pain in older adults with knee osteoarthritis: a randomized controlled pilot study. J Clin Neurosci. 1(77):128–133
Taylor AG, Anderson JG, Riedel SL, Lewis JE, Kinser PA, Bourguignon C (2013) Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 14(4):327–335
Hargrove JB, Bennett RM, Simons DG, Smith SJ, Nagpal S, Deering DE (2012) A randomized placebo-controlled study of noninvasive cortical electrostimulation in the treatment of fibromyalgia patients. Pain Med. 13(1):115–124
Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S (2018) Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia: a systematic review. Ann Intern Med. 168(6):414–421
Shekelle P, Cook I, Miake-Lye IM, Mak S, Booth MS, Shanman R, Beroes JM (2018) The effectiveness and risks of cranial electrical stimulation for the treatment of pain, depression, anxiety, PTSD, and insomnia: a systematic review. In: Eff Risks Cranial Electr Stimul Treat Pain, Depress Anxiety, PTSD, Insomnia A Syst Rev. Department of Veterans Affairs, Veterans Health Administration
Rintala DH, Tan G, Willson P, Bryant MS, Lai ECH (2010) Feasibility of using cranial electrotherapy stimulation for pain in persons with parkinson’s disease. Parkinsons Dis. 2010:1
Tan G, Rintala DH, Jensen MP, Richards JS, Holmes SA, Parachuri R, Lashgari-Saegh S, Price LR (2013) Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi-site randomized controlled trial with a secondary 6-month open-label phase. J Spinal Cord Med. 34(3):285–296
Feighner JP, Brown SL, Olivier JE (1973) Electrosleep therapy: a controlled double blind study. J Nerv Ment Dis. 157(2):121–128
Mischoulon D, De Jong MF, Vitolo OV, Cusin C, Dording CM, Yeung AS, Durham K, Parkin SR, Fava M, Dougherty DD (2015) Efficacy and safety of a form of cranial electrical stimulation (CES) as an add-on intervention for treatment-resistant major depressive disorder: a three week double blind pilot study. J Psychiatr Res. 1(70):98–105
Data availability
Not applicable
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Flowchart: Flow diagram of the literature search strategy (DOCX 28 KB)
CRediT author statement
Michał Szymoniuk: conceptualization; methodology; investigation; data curation; writing—original draft preparation; writing—review and editing; Jia-Hsuan Chin: investigation; data curation; writing—original draft preparation; visualization; Łukasz Domagalski: investigation, data curation, writing—original draft preparation; Mateusz Biszewski: investigation, data curation, writing—original draft preparation; Katarzyna Jóźwik: investigation, data curation, writing—original draft preparation; Piotr Kamieniak: writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Szymoniuk, M., Chin, JH., Domagalski, Ł. et al. Brain stimulation for chronic pain management: a narrative review of analgesic mechanisms and clinical evidence. Neurosurg Rev 46, 127 (2023). https://doi.org/10.1007/s10143-023-02032-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02032-1