Abstract
Synchronous or metachronous growth of multiple tumors (≥ 2) is found in up to 20% of meningioma patients. However, biological as well as histological features and prognosis are largely unexplored. Clinical and histological characteristics were retrospectively investigated in 95 patients harboring 226 multiple meningiomas (MMs) and compared with 135 cases of singular meningiomas (SM) using uni- and multivariate analyses. In MM, tumors occurred synchronously and metachronously in 62% and 38%, respectively. WHO grade was intra-individually constant in all but two MMs, and histological subtype varied in 13% of grade 1 tumors. MM occurred more commonly in convexity/parasagittal locations, while SM were more frequent at the skull base (p < .001). In univariate analyses, gross total resection (p = .014) and high-grade histology in MM were associated with a prolonged time to progression (p < .001). Most clinical characteristics and rates of high-grade histology were similar in both groups (p ≥ .05, each). Multivariate analyses showed synchronous/metachronous meningioma growth (HR 4.50, 95% CI 2.26–8.96; p < .001) as an independent predictor for progression. Compared to SM, risk of progression was similar in cases with two (HR 1.56, 95% CI .76–3.19; p = .224), but exponentially raised in patients with 3–4 (HR 3.25, 1.22–1.62; p = .018) and ≥ 5 tumors (HR 13.80, 4.06–46.96; p < .001). Clinical and histological characteristics and risk factors for progression do not relevantly differ between SM and MM. Although largely constant, histology and WHO grade occasionally intra-individually vary in MM. A distinctly higher risk of disease progression in MM as compared to SM might reflect different underlying molecular alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common primary intracranial neoplasms and occur in multiple intracranial or spinal locations (≥ 2) in one individual in up to 20% [14, 19, 20, 31]. Etiology of MM growth, with either multiple distant lesions at the date of diagnosis (synchronous MM) or the development of spatially separated tumors during follow-up (metachronous MM), has been sparsely investigated. MMs are more commonly found following whole brain radiation therapy during childhood, e.g., due to leukemia, and in patients suffering from phacomatoses, especially neurofibromatosis (NF) 2. Pathophysiological mechanisms underlying tumor manifestations at different, distant sites remain largely obscure but eventually suggest both sporadic multiple tumor growth and leptomeningeal spread [19]. While shown to be associated with increased Ki67 proliferation indices [17], multifocal tumor growth is observed among meningiomas of all WHO grades [1]. However, comparative histopathological analyses of tumors arising in one individual are sparse [4]. Genetic and molecular alterations underlying MM growth are largely unexplored. Previous analyses in small series or case reports of MM revealed single mutations or chromosomal aberrations of NF2 or SMARCB1 as well as their monoclonal origin [10, 27, 30, 33]. In contrast, a recent study showed different intra-individual driver mutations in MM [10, 15, 23].
Beyond pathophysiology and pathogenesis, treatment of MM remains a key challenge during neuro-oncological care. Few previous studies showed increased recurrence rates in MM as compared to singular lesions, suggesting different biological behavior and worse overall prognosis [13, 20, 35]. In fact, disseminated tumor growth at different intracranial sites and multiple recurrences limit local treatment options such as microsurgical resection or radiosurgery. In addition, effective chemotherapeutical options for meningioma patients are lacking [5]. Hence, further characterization of the clinical course of patients with MM and identification of risk factors for progression are urgently needed.
In this series, we therefore analyzed histological and clinical characteristics of patients suffering from sporadic neuropathologically confirmed synchronous or metachronous meningiomas and additionally provide comparative analyses with individuals harboring singular intracranial lesions.
Materials and methods
Data collection
Imaging, medical, and operative reports from all patients who underwent surgery for neuropathologically confirmed meningioma in our department (Department of Neurosurgery, University Hospital Münster, Germany), between 1991 and 2018, were reviewed. MM was classified in case of ≥ 2 histopathologically confirmed meningiomas or meningioma-suspicious intracranial lesions at the date of index surgery (synchronous) or during follow-up (metachronous MM). Cases with multiple tumors arising from the resection cavity/dura attachment after surgery for a single lesion were not included. Radiological diagnosis of meningioma not subjected to surgery was established in patients with the history of at least one neuropathologically confirmed meningioma and distant, synchronously or metachronously growing contrast-enhancing, extra-axial lesions. In surgically treated cases, meningioma diagnosis and grading were performed according to the 2016 classification of brain tumors in all cases [18]. The following data was registered as described previously [2, 7]: patients’ sex and age at the time of index surgery, Karnofsky Performance Score (KPS, [11]) prior to index surgery and at the date of last follow-up, indication for surgery (primary or recurrent meningioma), tumor location (classified as “skull base,” “convexity” and “parasagittal/falcine,” “spinal” and “intraventricular”), administration of preoperative or adjuvant irradiation, and the extent of resection according to the Simpson classification system [25], dichotomously registered as gross and subtotal resection (GTR, Simpson I–III vs STR, Simpson ≥ IV, [2]) for each single lesion. For comparative analyses, a cohort of patients with singular meningiomas with a follow-up of at least 3 years and full availability of the included histological and clinical data was retrieved from the Muenster Meningioma Database [2, 7, 26] using computed randomized sampling (IBM SPSS Statistics, Version 28, IBM, Ehningen, Germany). No further restrictions were applied when defining the cohort.
Initial routine postoperative MRI was scheduled 3 months after surgery and, in case of an event-free course, repeated in 12- and 6-month intervals in grade 1 and 2/3 lesions, respectively. After a progression-free interval of 5 years, follow-up imaging intervals were extended to 24 months in grade 1 and 12 months in grade 2/3 lesions [5]. Contrast-enhanced CT scans were performed in patients with contra-indications against MRI, and imaging was analyzed by a team of at least one neurosurgeon and one radiologist. Recurrence/progression was registered for each tumor and diagnosed in case of any increase in tumor size beyond MRI- or CT-depending measurement range, while development of distant tumors qualified registration as metachronous MM. Time to progression was correspondingly calculated from the date of initial diagnosis of each lesion to the date of its progression.
Statistical analyses
Statistical calculations were performed using statistic software (IBM SPSS Statistics, Version 28, IBM, Ehningen, Germany). Data are characterized by standard statistics: Categorical variables are described by absolute and relative frequencies and compared by Fisher’s exact test, while continuous variables are described by median and range and compared by Mann–Whitney U test. Time to progression (TTP) was defined as the duration between index surgery of surgically treated meningiomas or initial diagnosis of non-surgically treated lesions and radiologically confirmed tumor recurrence/progression or, in case of an event-free follow-up, to the date of last follow-up. TTP was analyzed by the Kaplan–Meier method and compared by Log-rank tests.
Multivariate Cox regression analyses for TTP included patients’ age, sex (female = reference (ref)), tumor location (convexity = ref), histology (grade 1 = ref; vs high-grade (grade 2/3)), and the extent of resection (GTR = ref). The results are characterized by hazard ratios (HR), 95% confidence intervals (CIs), and Wald-test p values. All reported p values are two-sided and considered statistically significant when < 0.05. Data collection and scientific use were approved by the local ethics committee (Münster 2018–061-f-S) and in accordance with the principles of the Declaration of Helsinki (human rights).
Results
Using the above-described approach, of 1404 patients in the Muenster Meningioma Database, 95 individuals (7%) with sporadic multiple meningiomas, harboring a total of 226 lesions (mean: 2.4 tumors per patient), were identified, while 5 patients suffering from NF1/2 and 6 individuals who received cranial irradiation during childhood were excluded. The number of patients decreased as the number of tumors per patient increased (Fig. 1). One hundred forty-one lesions were synchronously diagnosed at the date of presentation, while 85 tumors appeared metachronously during follow-up (62% vs 38%). Median duration between index surgery and the development of the following metachronous, distant lesion was 38 months. 132 tumors were subjected to microsurgery (59%), followed by adjuvant irradiation in 38 cases (29%). During the further clinical course, irradiation for tumor relapse or progression was performed in 15 lesions. Six patients received peptide receptor radionuclide therapy (PRRT), mostly with 177Lu-DOTATATE, while the remaining patients were subjected to observation imaging. In Table 1, the left column summarizes baseline clinical data of patients with multiple meningiomas.
Histology and clinical characteristics of patients with MM
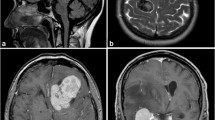
High-grade histology was diagnosed in 18 of the operated 132 MM (14%) and was found in nine of 85 synchronous and nine of 47 metachronous lesions (11% vs 19%, p = 0.192). Similarly, high-grade histology was not related with tumor location (p = 0.099) or KPS at the time of initial or last presentation (p = 0.295). However, mean KPS at the date of the last follow-up was lower in patients with high-grade meningiomas than in individuals with benign lesions (80, SD ± 20 vs 90, SD ± 10 vs, p = 0.025). Noteworthy, neuropathological analyses revealed a constant WHO grade among all analyzed samples except two patients. Those included two male patients, one suffering from six tumors, subsuming one benign, three high-grade, and two non-operated meningiomas (see illustrative Fig. 2), and another male suffering from three meningiomas, subsuming one benign, one high-grade, and one not operated lesion. In 56 samples of 25 patients with multiple grade 1 meningiomas who underwent surgery, neuropathological analyses further showed intra-individually similar and different histological subtypes of the analyzed specimen in 49 (87%) and 7 (13%) lesions, respectively.
Illustrative sample of a patient developing metachronous spatially distinct meningiomas. After resection of a left frontal parasagittal meningioma (a, axial T1-weighted, contrast-enhanced imaging), microscopic analyses revealed transitional meningioma, WHO grade 1, b). Four years later, the patient developed a left parietal, parasagittal lesion (axial T1-weighted, contrast-enhanced imaging, c, arrow), diagnosed as atypical meningioma (d, hematoxylin and eosin staining, each)
Risk factors for recurrence/progression
Within a median follow-up of 56 months (range: 47–64 months), tumor progression occurred in 34 of all operated and non-operated 226 MMs (15%). Progression was observed in 30 of 132 surgically treated lesions but in four of 90 lesions initially subjected to follow-up imaging alone (23% vs 4%, p < 0.001). Mean KPS at the date of last follow-up was 70 (SD ± 20) in patients with and 90 (SD ± 20) without tumor progression (p = 0.002). Progression was observed in 21 of 180 tumors in females but in 13 of 46 lesions in male patients (12% vs 28%, p = 0.010). No correlations were found between progression and age at diagnosis (p = 0.662). Progression rates (11% vs 21%, p = 0.055) and TTP (medians: 164 months vs not reached, p = 0.131) were similar comparing synchronously and metachronously diagnosed meningiomas. Among the 132 operated cases, the risk of progression did not significantly differ comparing GTR and STR (21% vs 43%, p = 0.091), while the median TTP was shorter after STR than after GTR (74 vs 111 months, p = 0.014, Fig. 3a). Progression was observed in 24 of 113 convexity (21%), 2 of 20 parasagittal/falcine (10%), 7 of 87 skull base (8%), and 1 of 6 spinal meningiomas (17%, p = 0.048). Moreover, 72% of the high-grade but 15% of the grade 1 meningiomas developed disease progression (N = 13 of 18 vs 17 of 114, p < 0.001), and TTP was shorter in grade 2/3 than in grade 1 lesions (13 vs 164 months, p < 0.001, log rank test, Fig. 3b). Multivariate, patient-related analyses of the 95 individuals with MM confirmed high-grade histology as the only independent predictor for disease progression (HR 8.11, 95% CI 3.03–21.67; p < 0.001, Table 2).
Comparative analyses of patients with MM with SM
Table 1, middle column, summarizes baseline clinical and histopathological data of the cohort of patients with SM (N = 135). Frequency of high-grade histology was 7% in skull base meningiomas (N = 5 of 76) but 18%, 22%, 13%, and 100% in convexity (N = 7 of 38), parasagittal/falcine (N = 5 of 23), spinal (N = 1 of 8), and intraventricular (N = 1 of 1) tumors, respectively (p = 0.024). High-grade histology was also more commonly found in males (N = 13 of 39, 33%, males, vs 4 of 96, 4%, females; p < 0.001), and not related with KPS prior index surgery (p = 0.174) or at the time of last follow-up (p = 0.139).
In comparative patient-related analyses (Table 1, right column), patients with MM were slightly older (75 vs 62 years, p = 0.011), while sex distribution (p = 0.165), the extent of resection (p = 0.576), and the mean KPS at the dates of presentation (p = 0.161) and last follow-up (p = 0.439) did not significantly differ between patients with SM and MM. Tumor locations differed significantly comparing MM and SM (p < 0.001, Table 1). Noteworthy, histopathological analyses revealed a similar distribution of grade 1 and 2/3 histology comparing MM and SM (p = 0.857). In contrast to SM, MM were lacking angiomatous, microcystic, psammomatous, chordoid, or anaplastic histology (Table 1).
Within a median follow-up of 78 months (range: 37–252 months), progression was observed in 21% of the patients with SM (N = 29). Risk factors for progression in SM are summarized in Table 2, middle column, and were similar compared to MM.
Cumulative multivariate analyses of SM together with MM adjusted for age, sex, tumor location, the extent of resection, and the WHO grade of the tumors further showed high-grade histology (HR 6.06, 95% CI 3.20–11.47; p < 0.001), synchronous or metachronous meningioma growth (HR 4.50, 95% CI 2.26–8.96; p < 0.001) and, with borderline significance, STR (HR 2.38, 95% CI 0.97–5.83; p = 0.059) as independent predictors for progression. Correspondingly, median TTP was distinctly lower in patients with multiple as compared to cases with singular lesions (164 vs 242 months, p = 0.011, Log rank test, Fig. 4). Further Cox regression analyses revealed a similar risk of recurrence in patients with singular and two (HR 1.56, 95% CI 0.76–3.19; p = 0.224) meningiomas, but an exponentially increasing risk of progression in patients with 3–4 lesions (HR 3.25, 1.22–1.62; p = 0.018) ≥ 5 tumors (HR 13.80, 4.06–46.96; p < 0.001).
Discussion
Clinical characteristics and histology of patients with MM
Frequency of MM (≥ 2) in our cohort was 7%, which matches the broad range of multiple meningioma incidence reported in previous series [6, 8, 19, 29, 31]. Median age in patients with MM in our series was 62 years and therefore comparable to prior reports [8, 31], but slightly higher as compared to patients with SM. With 79% of the patients, the female predominance in the MM cohort appeared to be higher as in patients harboring SM. Although without reaching the level of statistical significance, this observation matches findings from a previous study [19]. Similar to a former report [31], the majority of MM in our cohort were diagnosed synchronously at the date of initial presentation. As shown in Fig. 1, the frequency of cases with multiple lesions exponentially decreased as the number of tumors increased [31]. MM in our series were found more often in non-skull base locations as compared to SM, contradicting findings of a previous series [19], and further underlying the need of additional studies to define clinical characteristics of MM, regardless of the clinic’s corresponding specialization, e.g., on skull-base surgery. Further clinical characteristics of patients with MM and SM did not significantly differ, consistent with findings of Maiuri et al. [17].
High-grade histology was found in 13% of all operated MM in our study, which was in line with the control cohort of SM and with rates reported in previous studies on MM [1, 31]. In consideration of the rates of high-grade histology in meningiomas in general, these data suggest a similar distribution of grade 1 and 2/3 tumors in SM and MM. However, by nature, histology was only available in the subgroup of operated tumors, while the WHO grade of lesions treated with irradiation or observation remains obscure. This finding is remarkable as tumor spreading in general is usually assumed to be associated with malignancy and increased proliferation [17]. In contrast to findings in meningiomas in general and to our control cohort of SM [9, 12, 16], high-grade histology in MM was not associated with non-skull base tumor location. Previous studies revealed correlations between molecular characteristics and anatomical distributions of meningiomas in general [16, 22, 28, 34], further raising the questions of genetic and epigenetic differences between SM and MM. Moreover, mean KPS at the date of last follow-up was slightly lower in high-grade than in benign lesions, eventually reflecting disease progression and/or therapy side effects. Although the WHO grade of the tumors remained consistent in most patients, histological subtype differed intra-individually in a considerable portion. In a previous series of seven patients, histological subtype remained identical in specimen from different tumors [6]. In contrast, different histological subtypes of MM in one individual has been reported in other series [8, 31] and contradicts the thesis of direct arachnoid tumor spreading during pathogenesis of MM.
Prognosis of MM
Progression of MM was observed in 16% and was associated with a reduced KPS at the date of last follow-up. Similar to observations in meningiomas in general, rates of progression were higher in males than in females, eventually reflecting a higher incidence of high-grade histology among the first [24]. Correspondingly, no correlation between patients’ sex and recurrence in patients with MM was found in sex-adjusted multivariate analyses. The impact of the extent of resection on progression in MM is largely unexplored. In our MM cohort, despite similar progression rates, STR was related with shorter progression-free survival, matching findings from Ramos-Fresnedo et al. [19]. As this did not hold true in multivariate analyses, the efficacy of GTR on tumor progression in MM remains unclear. Correlation of STR with progression was also lacking in our control cohort of SM, although, in the entire data base, this association has been largely reported [2, 26, 32]. While we cannot exclude a bias, e.g., due to the low number of samples, biological behavior and tumor burden rather than the extent of resection might contribute to tumor progression in MM [3]. Even more than in SM, high-grade histology was found a strong predictor for progression in MM. Considering a lower impact of the extent of resection on progression in these lesions, this finding underlines the necessity of potent adjuvant treatment options in patients with MM and might also display a different biological behavior as compared to SM. This thesis is further underlined by our finding of a more than fourfold increased risk of progression of MM as compared SM. A longer follow-up of patients with SM as compared to patients with MM in our series further supports this theory. Although one might argue that an increasing probability of progression with the number of lesions at risk appears logically, the exponential increase is unexpected and contradicts this explanation. Analyses further elucidating underlying molecular alterations in MM, however, remain sparse. Small series and case reports showed mutations or chromosomal losses of NF2 or SMARCB1 in MM, as well as distinct somatic mutations in samples of different meningiomas of one individual [10, 30, 33]. Thus, future analyses are needed to enable molecular characterization of MM and to improve the understanding of their pathogenesis.
The authors are aware of some limitations of the study. Although providing analyses in a large series, our study suffers typical shortcomings from retrospective studies, e.g., selection bias. In addition, complexity of treatment of individual patients with MM causes data heterogeneity and could not be sufficiently considered in statistical analyses. For the same reason, further imaging data could not be subjected to statistical analyses. The number of patients suffering from extensive tumor burden (e.g., > 5 tumors) was low, potentially limiting interpretation of our results. Eventually, treated tumors display different biological behavior and characteristics (e.g., size) as compared to lesions simply subjected to observation, and cumulative analyses might have led to additional bias. Further details about irradiation were not sufficiently available and therefore not subjected to statistical analyses. Finally, samples were neuropathologically diagnosed according to the 2016 WHO classification of brain tumors [18], and molecular characteristics as suggested in the current WHO classification [21] as well as Ki67 labeling index were not considered.
In conclusion, patients with MM were found to lack distinct clinical characteristics as compared to SM. A potential predominant location of MM in non-skull base positions remains to be further investigated. Noteworthy, rates of high-grade tumors did not differ from SM, suggesting alternative underlying, e.g., molecular alterations promoting multiple tumor growth. Risk of progression in MM was generally increased as compared to SM, and exponentially raised with the number of lesions per patient.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Araujo Pereira BJ, Nogueira de Almeida A, Pires de Aguiar PH, Paiva WS, Teixeira MJ, Nagahashi Marie SK (2019) Multiple intracranial meningiomas: a case series and review of the literature. World Neurosurg 122:e1536–e1541. https://doi.org/10.1016/j.wneu.2018.11.097
Brokinkel B, Spille DC, Brokinkel C, Hess K, Paulus W, Bormann E, Stummer W (2021) The Simpson grading: defining the optimal threshold for gross total resection in meningioma surgery. Neurosurg Rev 44:1713–1720. https://doi.org/10.1007/s10143-020-01369-1
Brokinkel B, Spille DC, Schipmann S, Hess K, Paulus W, Stummer W (2021) Letter to the editor: “Surgery for recurrent meningiomas: the minor prognostic role of the extent of resection.” World Neurosurg 145:514–516. https://doi.org/10.1016/j.wneu.2020.09.092
Darshan HR, Patel BK, Singh A, Nair P, Poyuran R, Easwer HV (2021) Simultaneous trigonal and spinal meningioma with varied histology: a rare case report. Surg Neurol Int 12:611. https://doi.org/10.25259/SNI_1051_2021
Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, Preusser M, Minniti G, Lund-Johansen M, Lefranc F, Houdart E, Sallabanda K, Le Rhun E, Nieuwenhuizen D, Tabatabai G, Soffietti R, Weller M (2021) EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol 23:1821–1834. https://doi.org/10.1093/neuonc/noab150
Heinrich B, Hartmann C, Stemmer-Rachamimov AO, Louis DN, MacCollin M (2003) Multiple meningiomas: Investigating the molecular basis of sporadic and familial forms. Int J Cancer 103:483–488. https://doi.org/10.1002/ijc.10840
Hess K, Spille DC, Adeli A, Sporns PB, Brokinkel C, Grauer O, Mawrin C, Stummer W, Paulus W, Brokinkel B (2018) Brain invasion and the risk of seizures in patients with meningioma. J Neurosurg 130:789–796. https://doi.org/10.3171/2017.11.JNS172265
Huang H, Buhl R, Hugo HH, Mehdorn HM (2005) Clinical and histological features of multiple meningiomas compared with solitary meningiomas. Neurol Res 27:324–332. https://doi.org/10.1179/016164105X39932
Hwang WL, Marciscano AE, Niemierko A, Kim DW, Stemmer-Rachamimov AO, Curry WT, Barker FG 2nd, Martuza RL, Loeffler JS, Oh KS, Shih HA, Larvie M (2016) Imaging and extent of surgical resection predict risk of meningioma recurrence better than WHO histopathological grade. Neuro Oncol 18:863–872. https://doi.org/10.1093/neuonc/nov285
Juratli TA, Prilop I, Saalfeld FC, Herold S, Meinhardt M, Wenzel C, Zeugner S, Aust DE, Barker FG 2nd, Cahill DP, Brastianos PK, Santagata S, Schackert G, Pinzer T (2021) Sporadic multiple meningiomas harbor distinct driver mutations. Acta Neuropathol Commun 9:8. https://doi.org/10.1186/s40478-020-01113-2
Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: CM M (ed) Evaluation of chemotherapeutic agents. Columbia University Press, New York, New York, pp 191–205.
Lin BJ, Chou KN, Kao HW, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY (2014) Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121:1201–1208. https://doi.org/10.3171/2014.7.JNS132359
Liu F, Qian J, Ma C (2021) MPscore: a novel predictive and prognostic scoring for progressive meningioma. Cancers (Basel) 13. https://doi.org/10.3390/cancers13051113
Lusins JO, Nakagawa H (1981) Multiple meningiomas evaluated by computed tomography. Neurosurgery 9:137–141. https://doi.org/10.1227/00006123-198108000-00004
Lyu J, Quan Y, Wang JB, Gong SP (2020) Whole exome sequencing of multiple atypical meningiomas in a patient without history of neurofibromatosis type II: a case report. Am J Case Rep 21:e923928. https://doi.org/10.12659/AJCR.923928
Maiuri F, Mariniello G, Guadagno E, Barbato M, Corvino S, Caro DBD, M, (2019) WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir (Wien) 161:2553–2561. https://doi.org/10.1007/s00701-019-04084-z
Maiuri F, Mariniello G, Peca C, Guadagno E, Corvino S, d’Avanzo S, Caro BDDM, de Divitiis O (2020) Multicentric and diffuse recurrences of meningiomas. Br J Neurosurg 34:439–446. https://doi.org/10.1080/02688697.2020.1754335
Perry A, Louis DN, von Deimling A, Sahm F, Rushing EJ, Mawrin C, Claus EB, Loeffler J, Sadetzki S (2016) Meningiomas. In: Louis DN, Ohgaki H, Wiestler OD et al (eds) WHO classification of tumors of the central nervous system. International Agency on Cancer Research, Lyon, pp 232–245
Ramos-Fresnedo A, Domingo RA, Sanchez-Garavito JE, Perez-Vega C, Akinduro OO, Jentoft ME, Vora SA, Brown PD, Porter AB, Bendok BR, Link MJ, Middlebrooks EH, Trifiletti DM, Chaichana KL, Quinones-Hinojosa A, Sherman WJ (2021) The impact of multiple lesions on progression-free survival of meningiomas: In: Neurosurg J (ed) a 10-year multicenter experience. pp 1–9. https://doi.org/10.3171/2021.8.JNS211252.
Ramos-Fresnedo A, Domingo RA, Vivas-Buitrago T, Lundy L, Trifiletti DM, Jentoft ME, Desai AB, Quinones-Hinojosa A (2020) Multiple meningiomas: does quantity matter? a population-based survival analysis with underlined age and sex differences. J Neurooncol 149:413–420. https://doi.org/10.1007/s11060-020-03620-7
Sahm F, Brastianos PK, Claus EB, Mawrin C, Perry A, Santagata S, Von Deimlig A (2021) Meningioma. In: Brat DJ, Ellison DW, Figarella-Branger D et al (eds) Central nervous system tumours, 5th Edition. International Agency for Research on Cancer, Lyon (France), pp 284–297
Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, Okonechnikov K, Koelsche C, Reuss DE, Capper D, Sturm D, Wirsching HG, Berghoff AS, Baumgarten P, Kratz A, Huang K, Wefers AK, Hovestadt V, Sill M, Ellis HP, Kurian KM, Okuducu AF, Jungk C, Drueschler K, Schick M, Bewerunge-Hudler M, Mawrin C, Seiz-Rosenhagen M, Ketter R, Simon M, Westphal M, Lamszus K, Becker A, Koch A, Schittenhelm J, Rushing EJ, Collins VP, Brehmer S, Chavez L, Platten M, Hanggi D, Unterberg A, Paulus W, Wick W, Pfister SM, Mittelbronn M, Preusser M, Herold-Mende C, Weller M, von Deimling A (2017) DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol 18:682–694. https://doi.org/10.1016/S1470-2045(17)30155-9
Sheng HS, Shen F, Zhang N, Yu LS, Lu XQ, Zhang Z, Fang HY, Zhou LL, Lin J (2019) Whole exome sequencing of multiple meningiomas with varying histopathological presentation in one patient revealed distinctive somatic mutation burden and independent clonal origins. Cancer Manag Res 11:4085–4095. https://doi.org/10.2147/CMAR.S202394
Shin HK, Park JH, Cho YH, Kim YH, Hong SH, Kim JH, Roh SW, Jeon SR (2021) Risk factors for high-grade meningioma in brain and spine: systematic review and meta-analysis. World Neurosurg 151:e718–e730. https://doi.org/10.1016/j.wneu.2021.04.138
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39. https://doi.org/10.1136/jnnp.20.1.22
Spille DC, Hess K, Bormann E, Sauerland C, Brokinkel C, Warneke N, Mawrin C, Paulus W, Stummer W, Brokinkel B (2020) Risk of tumor recurrence in intracranial meningiomas: comparative analyses of the predictive value of the postoperative tumor volume and the Simpson classification. J Neurosurg 134:1764–1771. https://doi.org/10.3171/2020.4.JNS20412
Stangl AP, Wellenreuther R, Lenartz D, Kraus JA, Menon AG, Schramm J, Wiestler OD, von Deimling A (1997) Clonality of multiple meningiomas. J Neurosurg 86:853–858. https://doi.org/10.3171/jns.1997.86.5.0853
Sun C, Dou Z, Wu J, Jiang B, Iranmanesh Y, Yu X, Li J, Zhou H, Zhong C, Peng Y, Zhuang J, Yu Q, Wu X, Yan F, Xie Q, Chen G (2020) The preferred locations of meningioma according to different biological characteristics based on voxel-wise analysis. Front Oncol 10:1412. https://doi.org/10.3389/fonc.2020.01412
Terrier LM, Francois P (2016) Multiple meningiomas. Neurochirurgie 62:128–135. https://doi.org/10.1016/j.neuchi.2015.12.006
Torres-Martin M, Kusak ME, Isla A, Burbano RR, Pinto GR, Melendez B, Castresana JS, Rey JA (2015) Whole exome sequencing in a case of sporadic multiple meningioma reveals shared NF2, FAM109B, and TPRXL mutations, together with unique SMARCB1 alterations in a subset of tumor nodules. Cancer Genet 208:327–332. https://doi.org/10.1016/j.cancergen.2015.03.012
Tsermoulas G, Turel MK, Wilcox JT, Shultz D, Farb R, Zadeh G, Bernstein M (2018) Management of multiple meningiomas. J Neurosurg 128:1403–1409. https://doi.org/10.3171/2017.2.JNS162608
Voss KM, Spille DC, Sauerland C, Suero Molina E, Brokinkel C, Paulus W, Stummer W, Holling M, Jeibmann A, Brokinkel B (2017) The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J Neurooncol 133:641–651. https://doi.org/10.1007/s11060-017-2481-1
Wang AS, Jamshidi AO, Oh N, Sahyouni R, Nowroozizadeh B, Kim R, Hsu FPK, Bota D (2018) Somatic SMARCB1 mutation in sporadic multiple meningiomas: case report. Front Neurol 9:919. https://doi.org/10.3389/fneur.2018.00919
Youngblood MN, Duran D, Montejo JD, Li C, Omay SB, Sheth AH, Zhao AY, Tyrtova E, Miyagishima DF, Fomchenko EI, Hong CS, Clark VE, Riche M, Peyre M, Boetto J, Sohrabi S, Koljaka S, Baranoski JF, Knight J, Zhu H, Pamir MN, Avsar T, Kilic T, Schramm J, Timmer M, Goldbrunner R, Gong Y, Bayri Y, Amankulor N, Hamilton RL, Bilguvar K, Tikhonova I, Tomak PR, Huttner A, Simon M, Krischek B, Kalamarides M, Erson-Omay EZ, Moliterno J, Gunel M (2019) Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J Neurosurg 1–10. https://doi.org/10.3171/2019.8.JNS191266.
Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, Montejo JD, Zhao A, Sheth A, Tyrtova E, Ozduman K, Iacoangeli F, Peyre M, Boetto J, Pease M, Avsar T, Huttner A, Bilguvar K, Kilic T, Pamir MN, Amankulor N, Kalamarides M, Erson-Omay EZ, Gunel M, Moliterno J (2021) Associations of meningioma molecular subgroup and tumor recurrence. Neuro Oncol 23:783–794. https://doi.org/10.1093/neuonc/noaa226
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study conception: DCS, BB, OG. Data collection: LK, KH, CT, MM, BHA. Statistical analyses: BB, DCS. Study supervision: DCS, BB, WS, WP. Manuscript draft: LK, DCS, BB, MS, OG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Westfälische Wilhelms-Universität Münster and the Ärztekammer Westfalen-Lippe (Münster 2018–061-f-S).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not available.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopf, L., Warneke, N., Grauer, O. et al. Prognosis and histology of sporadic synchronous and metachronous meningiomas and comparative analyses with singular lesions. Neurosurg Rev 46, 55 (2023). https://doi.org/10.1007/s10143-023-01958-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-01958-w