Abstract
The effects of smoking on survival in BM patients have yet to be reviewed and meta-analysed. However, previous studies have shown that smokers had a greater risk of dying from lung cancer compared to non-smokers. This meta-analysis, therefore, aimed to analyse the effects of cigarette smoking on overall survival (OS) and progression-free survival (PFS) in lung cancer BM patients. PubMed, Embase, Web of Science, Cochrane and Google Scholar were searched for comparative studies regarding the effects of smoking on incidence and survival in brain metastases patients up to December 2020. Three independent reviewers extracted overall survival (OS) and progression-free survival data (PFS). Random-effects models were used to pool multivariate-adjusted hazard ratios (HR). Out of 1890 studies, fifteen studies with a total of 2915 patients met our inclusion criteria. Amongst lung carcinoma BM patients, those who were smokers (ever or yes) had a worse overall survival (HR: 1.34, 95% CI 1.13, 1.60, I2: 72.1%, p-heterogeneity < 0.001) than those who were non-smokers (never or no). A subgroup analysis showed the association to remain significant in the ever/never subgroup (HR: 1.34, 95% CI 1.11, 1.63) but not in the yes/no smoking subgroup (HR: 1.30, 95% CI 0.44, 3.88). This difference between the two subgroups was not statistically significant (p = 0.91). Amongst lung carcinoma BM patients, smoking was associated with a worse OS and PFS. Future studies examining BMs should report survival data stratified by uniform smoking status definitions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BMs) have the highest incidence of all central nervous system tumours in adult patients [1]. BMs disseminate most frequently from lung carcinomas (40–50%), breast carcinomas (15–25%) or melanoma (5–20%) [4, 15, 40]. BM survival has remained abysmal despite therapeutic and diagnostic advancements, leading to severe deterioration in function and quality of life [35].
Tobacco use, specifically cigarette smoking, is a major cause of preventable death and morbidity [39]. Smoking has been demonstrated as a risk factor for numerous cancers, including lung, liver and otolaryngological cancers. Both malignancy incidence and therapeutic response to anti-oncological agents are demonstrated to be altered due to smoking [42]. Previous studies examining the effect of smoking on cancer patients have often restricted the included patient population to non-metastatic patients [36, 37]. Smoking cessation may be less likely emphasised for many metastatic patients, especially those who are considered incurable and those with have limited expected survival time.
The effects of smoking on survival in BM patients have yet to be reviewed and meta-analysed. This meta-analysis, therefore, aimed to analyse the effects of cigarette smoking on overall survival (OS) and progression-free survival (PFS) in lung cancer BM patients.
Materials and methods
The systematic review was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist, and all steps of the PRISMA checklist were completed [34].
Inclusion and exclusion criteria
Our inclusion criteria aimed to identify comparative observational studies which analysed the effect of smoking on overall survival (OS) and/or progression-free survival (PFS) in lung cancer patients with brain metastases. If papers included more than one hazard ratio that met inclusion criteria, all would be included as long as the patient populations did not overlap. Search results were limited to English-language studies with > 10 participants.
Search strategy
The electronic databases MEDLINE (Pubmed), Web of Science, EMBASE, Cochrane and Google Scholar were searched using search terms related to smoking, intracranial metastases originating from lung carcinoma and patient survival (Supplementary materials, Appendix 1); studies published up until 31st December 2020 were included in our screening. Three independent authors (SC, IT, QZ) initially screened the title and abstracts against the inclusion and exclusion criteria. Subsequently, full text articles were retrieved and reviewed against eligibility criteria (SC, IT, QZ), with disagreements resolved through discussion with each other or a fourth author (AH).
Data extraction
Relevant data from each included study were extracted by three independent authors (SC, IT, QZ) as follows: (1) study characteristics, (2) cohort demographics, (3) smoking status, (4) primary tumour characteristics, (5) BM characteristics, (6) treatment characteristics and (7) outcomes (OS, PFS).
Quality assessment
Two independent authors (SC, QZ) assessed the quality of each included article using the Newcastle Ottawa Scale [46] for observational studies. Any disagreements were discussed and resolved amongst the authors; if consensus was not reached, a third author (IT) gave a final judgement. The Newcastle Ottawa Scale assesses the domains of subject selection (4 points), comparability (2 points) and assessment of outcome (3 points) for a total of 9 points. The score was interpreted as 0–3 points = “poor quality”, 4–6 points = “fair quality” and 7–9 points = “good quality”. Studies were penalised in the “selection of the non-exposed cohort” category if they lumped never and former smokers in one category when reporting exposure to smoking.
Data analysis
Statistical methods and analysis
We only included studies providing a multivariate hazard ratio (HR) in our main analysis. Pooled point estimates and their 95% confidence interval were calculated in the meta-analysis using the DerSimonian and Laird random-effects model [11] to account for inter-study variation. Subgroup analysis was performed to tease out heterogeneity in how smoking was reported across studies. Across the fifteen studies, smoking status was reported as either ever vs. never (12 studies) or yes vs. no (3 studies). If a study provided both intracranial and extracranial PFS as opposed to giving an overall PFS, only the results for intracranial PFS were included in the analysis to avoid patient overlap. Unless otherwise specified, a two-sided p value of < 0.05 was considered statistically significant. Data analysis was performed in RStudio v. 1.2.1335 (R Core Team, Vienna, Australia) using the package meta [3].
Sensitivity analysis
We performed sensitivity analyses which included (1) pooling the multivariate studies with the outlier removed and (2) pooling studies that provided a univariate HR. Similar to the multivariate analysis, a subgroup analysis was performed to compare studies reporting smoking as ever/never and yes/no.
Heterogeneity assessment and analysis
The degree of heterogeneity amongst studies was determined using the p value for the Cochrane Q test (statistically significant p value < 0.1) [20] and Higgins’ and Thompson’s I2 value [19]. Degree of heterogeneity was reported to be low, medium and high with I2 values of 25%, 50% and 75%, respectively [10].
Small study effect
Potential small study effects were identified using a funnel plot for visual determination of asymmetry, as well as Egger’s test for statistical significance [14]. When small study effects were indicated, the trim-and-fill method was used to impute the potentially missing studies and recalculate the imputed pooled effect estimate, whilst acknowledging the limitation of such a method, which assumes the source of asymmetry to be due solely to small study effect and not to other reasons.
Results
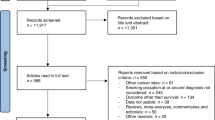
The search strategy returned 1890 articles following removal of duplicated papers. After title and abstract followed by full-text screening, fifteen studies [5, 6, 8, 12, 13, 18, 21, 23,24,25, 31, 32, 41, 50, 52] included data on multivariate HR (as opposed to univariate), meeting our inclusion criteria, and were included in our review and meta-analysis (Fig. 1). Papers reporting data on univariate HR [2, 8, 9, 12, 13, 16, 22,23,24,25, 29,30,31,32,33, 41, 42, 44, 48,49,50,51,52] (n = 23) were pooled in a sensitivity analysis and results can be found in supplementary materials. Of the 15 studies included, 13 were retrospective cohort studies, and two were prospective cohort studies. The mean study duration was 110.67 months, and the mean follow-up duration for OS and PFS was 17.11 months (Table 1). The most common covariates adjusted for in the multivariate models were age (n = 9) and sex (n = 7); the minimum number of covariates in a model was 3 [21, 26] and the maximum was 11 [32]. Of the total 3094 pooled participants, 63.2% (n = 1956) were male. Smoking status was reported in 87.0% (n = 2692) of patients—for the proportion that was reported, 64.2% (n = 1727) were current or past smokers and 35.8% (n = 965) had no smoking history. The histology of 48.2% (n = 1492) of the primary lung carcinomas was adenocarcinoma (Table 2).
Overall survival
Fifteen studies, with a total of 2915 patients with brain metastases, met our inclusion criteria. The pooled multivariate HR for overall survival was greater amongst smokers than non-smokers (HR: 1.34, 95% CI 1.13, 1.60; 15 studies, I2: 72.1%; p-heterogeneity: < 0.0001) (Fig. 2). This suggests that death rates in BM patients in the smoking group were 34% higher than those in BM patients in the non-smoking group. The HRs were found to have a high level of heterogeneity.
Forest plots showing pooled multivariate HR and 95% CI for all studies that compared overall survival comparing smokers vs. non-smokers lung carcinoma BM patients. Each study is shown by the point estimate of the hazard ratio and 95% confidence intervals (extending lines). The diamond centre represents the estimated pooled hazard ratio and width represents its 95% confidence interval (labelled total)
Subgroup analysis
A subgroup analysis was performed to tease out heterogeneity in how smoking was reported across studies. Studies reporting smoking as ever/never had significant results showing smoking to be associated with an increased risk of death (HR: 1.34, 95% CI: 1.11, 1.63; 12 studies; I2: 73%; p-heterogeneity < 0.01). Studies reporting smoking as yes/no also displayed that smoking increased the risk of death, but these results were not statistically significant (HR: 1.30, 95% CI: 0.44, 3.88; 3 studies; I2: 56%; p-heterogeneity: 0.10) (Fig. 3).
Progression-free survival
The pooled multivariate HR for PFS demonstrated worse outcomes amongst smokers (HR: 1.35, 95% CI 0.68, 2.68; 5 studies; I2: 80.8%; p-heterogeneity: 0.0003), when compared to non-smokers; however, this analysis did not reach statistical significance (supplementary materials Fig. 4) (Fig. 4). Stratifying by how smoking was reported was not feasible due to the paucity of studies in each category.
Sensitivity analysis
Rerunning our multivariate analysis excluding the outlier result (Li 2019) resulted in a pooled multivariate HR of 1.39 (95% CI 1.18, 1.64; 14 studies, I2 = 28.6%; p-heterogeneity: 0.15). This was similar to our results including the outlying HR (supplementary materials Fig. 1). Our sensitivity analysis pooling univariate studies yielded a pooled univariate HR of 1.14 (95% CI 1.05, 1.24; 23 studies, I2: 42%; p-heterogeneity: 0.02), which was consistent with our multivariate results (supplementary materials Fig. 2). Subgroup analysis was similarly consistent with the ever/never subgroup showing smoking to be associated with a significant increased risk of death (HR: 1.17, 95% CI 1.06, 1.30; 18 studies; I2: 53.1%; p-heterogeneity: < 0.01). Studies reporting smoking as yes/no showed that smoking was associated with a non-significant increase in death (HR: 1.05, 95% CI 0.88, 1.27; 7 studies; I2: 0%; p-heterogeneity: 0.55) (supplementary materials Fig. 3). Similarly, sensitivity analysis of PFS also suggested that smoking was associated with an increased risk of mortality (HR: 1.33, 95% CI 0.99, 1.79, I2: 70.1%; p-heterogeneity: 0.0027), but this was not statistically significant. Further subgroup analysis was not possible due to paucity of studies in each category.
Bias evaluation
Small study bias was present on visual examination of the funnel plots. Eggers’ test confirmed the presence of funnel plot asymmetry (p = 0.001) (Fig. 5). The trim-and-fill method was attempted using the random effects model. One study was trimmed. The adjusted hazard ratio significantly indicated that smoking was associated with an increased risk of death (HR: 1.30, 95% CI 1.11, 1.53). The quality score for the observational studies ranged from 6 to 8 out of a total of 9 points on the Newcastle–Ottawa Scale, with a median score of 7, indicating that the quality of the studies was borderline fair/good (Supplementary materials Table 1).
Discussion
This meta-analysis confirmed that smoking was statistically significantly associated with an increased risk of death in patients with brain metastases from lung cancer. Moreover, our subgroup analysis revealed that this result was consistent whether the smoking status was categorised as ever/never or yes/no. However, the yes/no subgroup yielded statistically insignificant results, which could be due to the small sample size (3 studies) or the fact that the “no” category could include patients who recently stopped smoking, leading to an underestimation of the associated risk.
Our findings are clinically significant for the management of patients with brain metastases. Previous studies and clinical empiricism have suggested that there is a lack of emphasis on smoking cessation for patients with brain metastases given their short life-expectancy [42].
This meta-analysis quantitatively assessed that BM patients that were non-smokers have longer median OS. Results for PFS did not reach significance, likely due to the smaller sample size. Several previous studies that examined the effect of smoking on cancer patients have restricted the examined cohort to non-metastatic patients [36, 37]. However, there is previous literature examining the effect of smoking on different types of metastases which have found similar results to ours [17, 27].
Our sensitivity analysis of univariate HR was consistent with our multivariate results. Controlling for other factors in the multivariate analysis resulted in the pooled effect size being more strongly suggesting the risk of death was greater amongst smokers. The overall quality assessment of the studies was noted to be “good”. However, it is important to note that three studies—Hendriks et al. (2019) [18], Inal et al. (2018) [21] and Kim et al. (2013) [24]—reported smoking status to be unknown for 14/255, 66/698 and 26/313 patients, respectively. Another key aspect limiting the quality of these studies is that smoking was self-reported by the patients and the fact that some studies did not explicitly categorise patients as current, former and never smokers, and instead lumped former and never smokers together.
Several biologic processes explain the link between smoking and poor outcomes in metastatic patients. Recently, a 2019 study found that smoking increased the incidence of brain metastases in lung cancer patients and that nicotine had a critical impact on promoting metastatic development by skewing microglia to alternatively activated phenotype and suppressing their role in innate immunity [47]. This ultimately enhances metastatic tumour growth. It is well-established that smoking alters biologic pathways of cancer resulting in greater proliferation, migration, invasion, angiogenesis and activation of pro-survival cellular pathways [42, 43]. This leads to a more malignant tumour phenotype and can therefore worsen outcomes of patients. This further supports our findings that smoking increases the risk of death in patients with brain metastases and stresses the importance of advocating smoking cessation for these patients.
Only three out of the 15 included studies reported on EGFR mutation status. Previous papers have found an association between smoking and a lower number of EGFR mutations [38, 45]. However, Tseng et al. [45] found that smokers had a shorter median overall survival (OS) amongst both EGFR-mutant and EGFR-wild type patients (17.8 vs. 21.1 months, and 7.9 vs. 11.4 months, respectively; both p < 0.001). If there is indeed a relation between smoking and EGFR mutation status, this suggests that we should not adjust for EGFR mutation as it is a downstream consequence of the exposure (smoking) and we would be over adjusting. Future studies should report on mutational status in order to further understand the relation of smoking to overall survival with respect to mutational status.
Additional questions must be addressed in order to achieve a more in-depth understanding of the impact of smoking on patients with lung cancer and metastases. Interestingly, a recent meta-analysis comparing lung cancer patients who quit smoking at or around diagnosis or during treatment to those who continued smoking concluded that quitting smoking was significantly associated with improved overall survival [7]. Future studies should report whether patients have quit smoking after diagnosis/around treatment in order to enable a meta-analysis that can analyse whether quitting smoking is indeed beneficial for patients with brain metastases. Notably, only four out of 15 studies [6] explicitly defined that overall survival counted cancer-related deaths only; future studies conduct a competing risk analysis where they report cause-specific hazards or sub-distribution hazards for cancer- or non-cancer-related causes of deaths [28]. Studies should also report the number of deaths from each cause.
Strengths and limitations
Limitations of this study include limiting our search to studies published in English. Additionally, there was heterogeneity in the way smoking status was reported (ever vs. never, yes vs. no) and we were unable to analyse the difference between current smokers and former smokers; nevertheless, we stratified our results by the way smoking categories were reported. Moreover, only two studies, Zhang et al. 2016 [50] and Kim et al. 2013 [24], provided data on current, past and never smoking. Heterogeneity in the definition of smoking status is an important limitation for future studies to address because the “no” category could include participants who were both never or former smokers, which can confound the estimated association. There was also a lack of consistent reporting of point estimates with only a portion of studies reporting multivariate HR along with univariate HR.
However, this study also had several strengths. We were able to demonstrate that smoking cessation improved survival outcomes in patients with lung carcinoma brain metastases. A strict protocol was adhered to in performing the meta-analysis. We were meticulous in extracting MV-adjusted HRs in our main analysis and considered univariate HRs in a sensitivity analysis, as these would be biassed. Additionally, we teased out the heterogeneity in which smoking was reported. Furthermore, we analysed 15 studies with a sample size of 3094, which allowed good power in detecting a statistically significant hazard ratio, which favoured non-smokers.
Conclusion
In conclusion, our meta-analysis suggests that a smoking history has detrimental effects even in a progressive phase of malignancy. There were similar results for PFS but this did not reach statistical significance. Future studies should use a standardised way of reporting smoking status, such as never, past and current smokers, to facilitate analysis on how smoking cessation after diagnosis impacts survival. Future studies should document whether patients have quit smoking after diagnosis/around treatment in order to determine if quitting smoking is indeed beneficial for patients with brain metastases. Additionally, a time-to-event analysis would be beneficial to compare brain metastases between never, past and current smokers.
Supplementary information.
Data availability
The review data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The review data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Amsbaugh MJ, Kim CS (2022) Brain metastasis. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470246/
An N, Wang H, Li J, Zhai X, Jing W, Jia W, Kong L, Zhu H, Yu J (2019) Therapeutic effect of first-line EGFR-TKIs combined with concurrent cranial radiotherapy on NSCLC patients with EGFR activating mutation and brain metastasis: a retrospective study. Onco Targets Ther 12:8311. https://doi.org/10.2147/OTT.S223216
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial 22:153–160. https://doi.org/10.1136/EBMENTAL-2019-300117
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Byeon S, Ham JS, Sun J-M, Lee S-H, Ahn JS, Park K, Ahn M-J (2016) Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non-small cell lung cancer and brain metastasis. Med Oncol 33. https://doi.org/10.1007/S12032-016-0811-3
Cai L, Zhu JF, Zhang XW, Lin SX, Su XD, Lin P, Chen K, Zhang LJ (2014) A comparative analysis of EGFR mutation status in association with the efficacy of TKI in combination with WBRT/SRS/surgery plus chemotherapy in brain metastasis from non-small cell lung cancer. J Neurooncol 120:423–430. https://doi.org/10.1007/S11060-014-1570-7
Caini S, Del Riccio M, Vettori V, Scotti V, Martinoli C, Raimondi S, Cammarata G, Palli D, Banini M, Masala G, Gandini S (2022) Quitting smoking at or around diagnosis improves the overall survival of lung cancer patients: a systematic review and meta-analysis. J Thorac Oncol 17:623–636. https://doi.org/10.1016/J.JTHO.2021.12.005/ATTACHMENT/BD4703CD-A5A3-414C-9326-B396013042D9/MMC2.DOC
Chen CH, Lee HH, Chuang HY, Hung JY, Huang MY, Chong IW (2019) Combination of whole-brain radiotherapy with epidermal growth factor receptor tyrosine kinase inhibitors improves overall survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Cancers (Basel) 11. https://doi.org/10.3390/CANCERS11081092
Chen K, Zhang F, Fan Y, Cheng G (2020) Lung-molgpa index predicts survival outcomes of non-small-cell lung cancer patients with synchronous or metachronous brain metastases. Onco Targets Ther 13:8837–8844. https://doi.org/10.2147/OTT.S255478
Chen Y, Chirikov VV, Marston XL, Yang J, Qiu H, Xie J, Sun N, Gu C, Dong P, Gao X (2020) Machine learning for precision health economics and outcomes research (P-HEOR): conceptual review of applications and next steps. J Heal Econ Outcomes Res 7:35–42
DerSimonian R, Laird N, R D, N L, (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Du TQ, Li X, Zhong WS, Tian JD, Zhao YX, Liu D (2021) Brain metastases of lung cancer: comparison of survival outcomes among whole brain radiotherapy, whole brain radiotherapy with consecutive boost, and simultaneous integrated boost. J Cancer Res Clin Oncol 147:569–577. https://doi.org/10.1007/S00432-020-03359-8
Duell T, Kappler S, Knöferl B, Schuster T, Hochhaus J, Morresi-Hauf A, Huber RM, Tufman A, Zietemann V (2015) Prevalence and risk factors of brain metastases in patients with newly diagnosed advanced non-small-cell lung cancer. Cancer Treat Commun 4:106–112. https://doi.org/10.1016/j.ctrc.2015.08.004
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629. https://doi.org/10.1136/bmj.315.7109.629
Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK (2011) The biology of brain metastases—translation to new therapies. Nat Rev Clin Oncol 8:344. https://doi.org/10.1038/NRCLINONC.2011.58
Enders F, Geisenberger C, Jungk C, Bermejo JL, Warta R, Von Deimling A, Herold-Mende C, Unterberg A (2016) Prognostic factors and long-term survival in surgically treated brain metastases from non-small cell lung cancer. Clin Neurol Neurosurg 142:72–80. https://doi.org/10.1016/J.CLINEURO.2016.01.011
Foerster B, Pozo C, Abufaraj M, Mari A, Kimura S, D’Andrea D, John H, Shariat SF (2018) Association of smoking status with recurrence, metastasis, and mortality among patients with localized prostate cancer undergoing prostatectomy or radiotherapy: a systematic review and meta-analysis. JAMA Oncol 4:953–961. https://doi.org/10.1001/JAMAONCOL.2018.1071
Hendriks LEL, Henon C, Auclin E, Mezquita L, Ferrara R, Audigier-Valette C, Mazieres J, Lefebvre C, Rabeau A, Le Moulec S, Cousin S, Duchemann B, le Pechoux C, Botticella A, Ammari S, Gazzah A, Caramella C, Adam J, Lechapt E, Planchard D, De Ruysscher D, Dingemans AM, Besse B (2019) Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol 14:1244–1254. https://doi.org/10.1016/J.JTHO.2019.02.009
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/BMJ.327.7414.557
Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J (2006) Assessing heterogeneity in meta-analysis: I2 or Q statistic? Psychol Methods 11:193–206
Inal A, Kodaz H, Odabas H, Duran A, Seker M, Inanc M, Elkiran E, Gunaydin Y, Menekse S, Topcu T, Urakci Z, Tastekin D, Bilici M, Cihan S, Geredeli C, Sezer E, Uncu D, Arpaci E, Ozturk B, Bal O, Uysal M, Tanriverdi O, Gumus M, Oven Ustaalioglu B, Suner A, Cokmert S, Hacibekiroglu I, Aydin K, Isikdogan A (2018) Prognostic factors of patients who received chemotherapy after cranial irradiation for non-small cell lung cancer with brain metastases: a retrospective analysis of multicenter study (Anatolian Society of Medical Oncology). J Cancer Res Ther 14:578–582. https://doi.org/10.4103/0973-1482.176417
Jiang T, Zhang Y, Li X, Zhao C, Chen X, Su C, Ren S, Yang N, Zhou C (2019) EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer 121:98–108. https://doi.org/10.1016/J.EJCA.2019.08.021
Kim IA, Lee JS, Kim HJ, Kim WS, Lee KY (2018) Cumulative smoking dose affects the clinical outcomes of EGFR-mutated lung adenocarcinoma patients treated with EGFR-TKIs: a retrospective study. BMC Cancer 18. https://doi.org/10.1186/S12885-018-4691-0
Kim J, Lee SM, Yim JJ, Yoo CG, Kim YW, Han SK, Yang SC (2013) J K, SM L, JJ Y, CG Y, YW K, SK H, SC Y. Prognosis for non-small cell lung cancer patients with brain metastases 4:167–173. https://doi.org/10.1111/J.1759-7714.2012.00164.X
Kobayashi H, Hamasaki M, Morishita T, Yoshimura M, Nonaka M, Abe H, Inoue T, Nabeshima K (2018) Clinicopathological and genetic characteristics associated with brain metastases from lung adenocarcinoma and utility as prognostic factors. Oncol Lett 16:4243–4252. https://doi.org/10.3892/OL.2018.9225
Kobayashi SD, Malachowa N, Deleo FR (2015) Pathogenesis of Staphylococcus aureus abscesses. Am J Pathol 185:1518–1527. https://doi.org/10.1016/j.ajpath.2014.11.030
Kroeger N, Li H, De Velasco G, Donskov F, Sim HW, Stühler V, Wells JC, Stukalin I, Heide J, Bedke J, Agarwal N, Parekh H, Rini BI, Knox JJ, Pantuck A, Choueiri TK, Chin Heng DY (2019) Active smoking is associated with worse prognosis in metastatic renal cell carcinoma patients treated with targeted therapies. Clin Genitourin Cancer 17:65–71. https://doi.org/10.1016/J.CLGC.2018.09.006
Lau B, Cole SR, Gange SJ (2009) Competing risk regression models for epidemiologic data. Am J Epidemiol 170:244–256. https://doi.org/10.1093/AJE/KWP107
Lee H-H, Chen C-H, Chuang H-Y, Huang Y-W (2019) Huang M-Y (2019) Brain surgery in combination with tyrosine kinase inhibitor and whole brain radiotherapy for epidermal growth factor receptor-mutant non-small-cell lung cancer with brain metastases. Sci Reports 91(9):1–11. https://doi.org/10.1038/s41598-019-53456-z
Lee HL, Chung TS, Ting LL, Tsai JT, Chen SW, Chiou JF, Leung HW, Liu HE (2012) EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol 7. https://doi.org/10.1186/1748-717X-7-181
Li YD, Lamano JB, Kaur G, Lamano JB, Veliceasa D, Biyashev D, Kruser T, Bloch O (2019) Lymphopenia predicts response to stereotactic radiosurgery in lung cancer patients with brain metastases. J Neurooncol 143:337–347. https://doi.org/10.1007/S11060-019-03169-0
Lu F, Hou Y, Xia Y, Li L, Wang L, Cao K, Chen H, Chang L, Li W (2019) <p>Survival and intracranial control outcomes of whole-brain radiotherapy (WBRT) alone versus WBRT plus a radiotherapy boost in non-small-cell lung cancer with brain metastases: a single-institution retrospective analysis</p>. Cancer Manag Res 11:4255–4272. https://doi.org/10.2147/CMAR.S203461
Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD, Camidge DR, Braunstein SE, Boreta LC, Balasubramanian SK, Ahluwalia MS, Rana NG, Attia A, Gettinger SN, Contessa JN, Yu JB, Chiang VL (2017) Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol 35:1070–1077. https://doi.org/10.1200/JCO.2016.69.7144
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Park K, Bae GH, Kim WK, Yoo C-J, Park CW, Kim S-K, Cha J, Kim JW (2021) Jung J (2021) Radiotherapy for brain metastasis and long-term survival. Sci Reports 111(11):1–8. https://doi.org/10.1038/s41598-021-87357-x
Parsons A, Daley A, Begh R, Aveyard P (2010) Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 340:251. https://doi.org/10.1136/BMJ.B5569
Reitsma M, Kendrick P, Anderson J, Arian N, Feldman R, Gakidou E, Gupta V (2020) Reexamining rates of decline in lung cancer risk after smoking cessation. A meta-analysis Ann Am Thorac Soc 17:1126–1132. https://doi.org/10.1513/ANNALSATS.201909-659OC
Ren JH, He WS, Yan GL, Jin M, Yang KY, Wu G (2012) EGFR mutations in non-small-cell lung cancer among smokers and non-smokers: a meta-analysis. Environ Mol Mutagen 53:78–82. https://doi.org/10.1002/EM.20680
Samet JM (2013) Tobacco smoking: the leading cause of preventable disease worldwide. Thorac Surg Clin 23:103–112. https://doi.org/10.1016/J.THORSURG.2013.01.009
Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A, LJ S, J R, HA H, A T, (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–2705. https://doi.org/10.1002/CNCR.10541
Sekine A, Satoh H, Iwasawa T, Tamura K, Hayashihara K, Saito T, Kato T, Arai M, Okudela K, Ohashi K, Ogura T (2014) Prognostic factors for brain metastases from non-small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol 31:1–10. https://doi.org/10.1007/S12032-014-0228-9
Shenker RF, McTyre ER, Ruiz J, Weaver KE, Cramer C, Alphonse-Sullivan NK, Farris M, Petty WJ, Bonomi MR, Watabe K, Laxton AW, Tatter SB, Warren GW, Chan MD, Alphonse-Sullivan NK, Farris M, Petty WJ, Bonomi MR, Watabe K, Laxton AW, Tatter SB, Warren GW, Chan MD, Alphonse-Sullivan NK, Farris M, Petty WJ, Bonomi MR, Watabe K, Laxton AW, Tatter SB, Warren GW, Chan MD (2017) The effects of smoking status and smoking history on patients with brain metastases from lung cancer. Cancer Med 6:944. https://doi.org/10.1002/CAM4.1058
Sobus SL, Warren GW (2014) The biologic effects of cigarette smoke on cancer cells. Cancer 120:3617–3626. https://doi.org/10.1002/CNCR.28904
Sun H, Xu L, Wang Y, Zhao J, Xu K, Qi J, Yuan Z, Zhao L (2018) Wang P (2018) Additional radiation boost to whole brain radiation therapy may improve the survival of patients with brain metastases in small cell lung cancer. Radiat Oncol 131(13):1–7. https://doi.org/10.1186/S13014-018-1198-4
Tseng CH, Chiang CJ, Sen TJ, Yang TY, Hsu KH, Chen KC, Wang CL, Chen CY, Yen SH, Tsai CM, Huang MS, Ho CC, Yu CJ, Tsai YH, Chen JS, Chou TY, Tsai MH, Chen HY, Su KY, Chen JJW, Chen HW, Yu SL, Liu TW, Chang GC (2017) EGFR mutation, smoking, and gender in advanced lung adenocarcinoma. Oncotarget 8:98384. https://doi.org/10.18632/ONCOTARGET.21842
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, Luchini C, Stubbs B, Solmi M, Veronese N (2017) Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal 5(4):80–84
Wu SY, Xing F, Sharma S, Wu K, Tyagi A, Liu Y, Zhao D, Deshpande RP, Shiozawa Y, Ahmed T, Zhang W, Chan M, Ruiz J, Lycan TW, Dothard A, Watabe K (2020) Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function. J Exp Med 217. https://doi.org/10.1084/JEM.20191131
Wu YL, Zhou C, Cheng Y, Lu S, Chen GY, Huang C, Huang YS, Yan HH, Ren S, Liu Y, Yang JJ (2013) Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol Off J Eur Soc Med Oncol 24:993–999. https://doi.org/10.1093/ANNONC/MDS529
Yu X, Fan Y (2019) Real-world data on prognostic factors for overall survival in EGFR-mutant non-small-cell lung cancer patients with brain metastases. J Cancer 10:3486–3493. https://doi.org/10.7150/JCA.30292
Zhang Q, Zhang X, Yan H, Jiang B, Xu C, Yang J, Chen Z, Su J, Wu YL, Zhou Q (2016) Effects of epidermal growth factor receptor-tyrosine kinase inhibitors alone on EGFR-mutant non-small cell lung cancer with brain metastasis. Thorac cancer 7:648–654. https://doi.org/10.1111/1759-7714.12379
Zhu J, Wang W, Xu S, Jia C, Zhang Q, Xia Y, Wang W, Wen M, Wang X, Wang H, Zhang Z, Cai L, Zhang L, Jiang T (2020) Evaluation of the effect of lymph node status on the survival of non-small cell lung cancer patients with brain metastases: applications of a novel grade prognostic assessment score model involving N stage. Front Oncol 0:2273. https://doi.org/10.3389/FONC.2020.563700
Zhuang QY, Li JL, Lin FF, Lin XJ, -lin H, -Wang Y, -Lin Y, Huang YX, Zhang XQ, Tang LR, Wu JX, (2020) High biologically effective dose radiotherapy for brain metastases may improve survival and decrease risk for local relapse among patients with small-cell lung cancer: a propensity-matching analysis. Cancer Control 27:1–11. https://doi.org/10.1177/1073274820936287
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and/or design. Three independent authors (SC, IT, QZ) initially screened the title and abstracts against the inclusion and exclusion criteria. Subsequently, full text articles were retrieved and reviewed against eligibility criteria (SC, IT, QZ), with disagreements resolved through discussion with each other or a fourth author (AH). SC performed the statistical analysis under supervision of RM and MB. The first draft was written by SC, IT and QZ. Subsequently, RM and MB critically reviewed the revised versions of this manuscript afterward. SC and IT compiled the final draft. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval
This publication is a systematic review and meta-analysis in accordance with the PRISMA guidelines. No ethical approval is required.
Consent to participate
NA
Consent for publication
All authors consent to the publication of this meta-analysis in Neurosurgical Review.
Competing interests
The authors dcelare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shreya Chawla and Ishaan A. Tewarie are joint first authors.
Rania A. Mekary and Marike L.D. Broekman are joint senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chawla, S., Tewarie, I.A., Zhang, Q.O. et al. The effect of smoking on survival in lung carcinoma patients with brain metastasis: a systematic review and meta-analysis. Neurosurg Rev 45, 3055–3066 (2022). https://doi.org/10.1007/s10143-022-01832-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-022-01832-1