Abstract
In light of our own experiences, we value the existing literature to critically point out possible “near” future applications of optical coherence tomography (OCT) as an intraoperative neurosurgical guidance tool. “Pub Med”, “Cochrane Library”, “Crossref Metadata Search”, and “IEEE Xplore” databases as well as the search engine “Google Scholar” were screened for “optical coherence tomography + neurosurgery”, “optical coherence tomography + intraoperative imaging + neurosurgery”, and “microscope integrated optical coherence tomography + neurosurgery”. n = 51 articles related to the use of OCT as an imaging technique in the field of neurosurgery or neurosurgical research. n = 7 articles documented the intraoperative use of OCT in patients. n = 4 articles documented the use of microscope-integrated optical coherence tomography as a neurosurgical guidance tool. The Results demonstrate that OCT is the first imaging technique to study microanatomy in vivo. Postoperative analysis of intraoperative scans holds promise to enrich our physiological and pathophysiological understanding of the human brain. No data exists to prove that OCT-guided surgery minimizes perioperative morbidity or extends tumor resection. But results suggest that regular use of microscope-integrated OCT could increase security during certain critical microsurgical steps like, e.g., dural dissection at cavernous sinus, transtentorial approaches, or aneurysm clip placement. Endoscopy integration could aid surgery in regions which are not yet accessible to real-time imaging modalities like the ventricles or hypophysis. Theranostic instruments which combine OCT with laser ablation might gain importance in the emerging field of minimal invasive tumor surgery. OCT depicts vessel wall layers and its pathologies uniquely. Doppler OCT could further visualize blood flow in parallel. These abilities shed light on promising future applications in the field of vascular neurosurgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microneurosurgery remains an exceedingly demanding and dexterous fine motor task. Microscope-integrated three-dimensional imaging techniques which delineate the microstructural composition of tissue in the field of view are missing so far.
OCT imaging depends on the detection of back-scattered near-infrared light and is therefore harmless to biological tissue [3]. Notably OCT offers an outstanding axial spatial resolution from 1 to 15 μm. Among in vivo imaging methods, it remains unprecedented and approaches spatial resolution of conventional histopathology [10]. Penetrating depths depend on the optical tissue density. They range from 4 mm in air to 2.5 mm in dens tissue. With approximately 3.1 mm in the human cerebral cortex OCT well meets microsurgical requirements [9].
Physically depending on light microscope integration is fairly simple [25]. This opens up the ability of contact free three-dimensional, real-time scanning of tissue in the field of view during microsurgical procedures [26]. In ophthalmology, the technique yet proved robustness and is daily integrated in vitreoretinal surgical setups [19, 36].
In the neuroimaging domain, recent optical and image processing advancements like automatic serial sectioning of polarization-sensitive OCT (asPSOCT) and speckle modulation even increased image quality to such an extent that in vitro representation of cortical layers at single cell width were possible (see Fig. 1) [13, 29, 37, 41].
Recent technical developments in OCT. (1) Speckle-modulated OCT. Speckle artifacts limit the spatial to noise ratio in OCT imaging. These exemplary speckle-modulated OCT scans of the mouse cornea and retina show the increase of resolution in contrast to conventional OCT imaging. (1A) Conventional OCT scan of mouse cornea. (1B) Speckle modulated OCT scan of same mouse cornea, notice enhanced sectioning of histological layers. (1C, D) Enlarged excerpts (1D) notice enhanced delineation of histological structures like lamellae and enhanced delineation of the endothelium in speckle-modulated OCT. (1E) Histological section of cornea. (1F) Conventional OCT scan of mouse retina. (1G) Speckle-modulated OCT scan of mouse retina. (1H, I) Enlarged excerpts (1E) notice enhanced segregation of single retinal layers; see Yecies et al. [41]. (2) Polarization-sensitive OCT (ps-OCT). Through a set of hardware and software components, polarization-sensitive OCT (ps-OCT) is able to measure and correct the birefringence (“bi-refraction” of light) of local regions of tissue, leading to enhanced imaging of tissue with different optical densities and refraction indices. (2A) ps-OCT of a block of human cerebellar lobule. The folded cerebellar cortex is shown on orthogonal viewing planes (xy coronal; xz axial; yz sagittal). Note the ability to delineate the Purkinje cell layer. Volume rendering of segmented (2B) molecular layer, (2C) granual layer, and (2D) white matter (see Wang et al. [37]. (3) Doppler OCT. In vivo delineation of mouse cortical vasculature with Doppler OCT. (3A) Multi-photon laser scanning microscopy (MPM) of cerebral vasculature (3B) three-dimensional reconstruction of flow demonstrating the vasculature of the mouse cortex. (3C) Doppler OCT velocity projection map (see Gagnon et al. [13]. (4) Sensitivity contrast-enhanced OCT. Imaging of lymph vessels in ears pinnae in living mice. Injection of large gold nanorods LGNR is used for functional imaging. (4a) Delineation of blood vessels (red) by flow detection in OCT prior to LGNR injection. (4b) Injection of 815 nm LGNRs (green) and 925 nm LGNRS (cyan). (4c) Drainage of LGNRs and delineation of lymphatic vessels. (4d) Same imaging technique in a different mouse after injection of LGNRs (4e) enlarged excerpt displaying the relationship of blood and lymphatic vessels (4f) same area as in (4e) after injection of 925 nm LGNRs displaying the (arrow) junction of lymph vessels and mono directional flow (see Liba (2016))
OCT has the ability to perform an “optic biopsy”. Not only white and gray matter but also healthy and diffusely invaded brain tissue could be distinguished in glioma surgery [2, 12, 14, 24, 31].
A part from structural imaging, functional brain imaging is possible. Adaptations of perfusion-dependent OCT offer the possibility of functional cortical mapping after peripheral stimulation and furthermore the delineation of epileptic foci [32, 33, 35].
These versatile strengths shed light on OCTs potential for microneurosurgical guidance. This critical literature review focuses on clinical “near” future applications to further enhance neurosurgical excellence.
Materials and methods
“Pub Med”, “Cochrane Library”, “Crossref Metadata Search”, and “IEEE Xplore” databases as well as the search engine “Google Scholar” were screened for “optical coherence tomography + neurosurgery”, “optical coherence tomography + intraoperative imaging + neurosurgery”, and “microscope integrated optical coherence tomography + neurosurgery”.
Results
Detailed evaluation of the results revealed n = 51 articles related to the use of OCT as an imaging technique in neurosurgery or in the field of neurosurgical research. n = 7 articles documented the intraoperative use of OCT in patients. n = 4 articles documented the use of microscope-integrated optical coherence tomography as a neurosurgical guidance tool.
Discussion
Fundamental research
OCT allows to study microanatomy in vivo. Analysis of the human subarachnoid space with microscope-integrated OCT could delineate for the first time its intact microstructural composition. The arachnoid barrier cell membrane, trabecular system, inlying blood vessels, pia mater, and brain cortex could be well-delineated. OCT was further the first imaging modality to measure the height of these structures in vivo with an accuracy of 7.5 µm. Increased heights of the arachnoid barrier cell membrane at the Sylvian fissure manifested [17] (see Fig. 1).
Analysis of the cranial dura mater demonstrated differentiation of the outer periostal and inner meningeal layer as well as the microanatomical structure of mayor dural blood vessels like arteria meningea media with its vessel wall layers. Measurements of the cranial dura mater documented interindividual highly variable thicknesses [15] (see Fig. 1).
Extravascular OCT could delineate the microstructural composition of cerebral vessel walls with richness of detail [8, 38, 42]. It proved to delineate tunica interna, media, externa, and adventitia in cerebral arteries. Clinical relevant pathologies like calcifications and arteriosclerosis could be further displayed. Scanning of an incidental vasospasm could well define contraction of tunica media with increased thickness and decreased luminal diameters (preliminary results of our research group). The amorphous character of cerebral aneurysm walls with residual tunica media could be delineated for the first time [18] (see Fig. 1).
Intra-axial lesions
Primary applications of OCT in neurosurgery focused on the ability to distinguish healthy and tumor-infiltrated brain tissue. This ability could be demonstrated in glial tumors as well as malignant melanomas in vitro and later in vivo [2, 4, 5, 24, 27, 34]. Further technical development of cross-polarization OCT even enhanced such tumor detection qualities [22, 39, 40]. Aside from experimental setups, OCT proved these abilities during human glioma resection [1, 14]. Factors which might limit the use of OCT during high-grade glioma surgery might be extended globe shaped resection cavities which need multiple adaptations of the microscope angle to acquire reliable orthograde scans. Another factor could be the diffuse infiltrative growth which often leads to functional-based rather than tumor margin-based resections. Thirdly, 5-ALA states a well-established tool in this domain {Stummer:2006ib}.
Needle interventions
Due to its physical properties, integration into optical devices is fairly simple [5]. Needle integration aided and controlled the placement of epidural catheters in a porcine model [23]. A lateral viewing probe could discriminate blood vessels at biopsy site in human brain tumors [34]. Since OCT can distinguish between gray and white matter, fine placement of electrodes for deep brain stimulation could be guided in a rodent model [28, 30]. A combination with laser ablation systems has the ability of direct real-time feedback to guide the ablation process in a porcine brain tumor model [7, 11, 21]. These yet experimental theranostic instruments could be promising in the advancing field of minimal invasive tumor, radiant necrosis, and epileptic surgery.
Extra-axial lesions
OCT-guided dissection of cranial dura mater showed the ability to discriminate dural layers. Thin dura mater in combination with low optical density enabled transdural OCT scanning. These scans showed a sufficient image quality to delineate concealed microanatomical structures like the subarachnoid space, inlying blood vessels, or the brain cortex [15] (see Fig. 1). No literature exists on the use of OCT during meningioma surgery. The above mentioned study suggests that OCT would only be suitable to delineate crucial venous structures like the sinus in certain meningiomas with a low optical density.
Vascular neurosurgery
Microscope-integrated OCT could well delineate the microstructural composition of cerebral vessel walls [8, 38, 42] (Fig. 2). Clinical relevant characteristics like wall thickness, different layers, calcifications, and arteriosclerosis could be clearly defined in cerebral arteries, veins, and aneurysms [18]. These promising results should lead to further studies in the field of neurovascular surgery like bypass surgery [20].
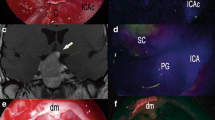
Microscope Integrated OCT. A Light microscopic image after right fronto-lateral craniotomy, during dissection of dura mater. Opened segment shows Sylvian fissure with superficial Sylvian veins and temporal as well as frontal brain cortex. Orange line indicates region of scan. B OCT scan of dura mater depicting the (1) outer endosteal and (2) inner meningeal layer. Strikingly, a (3) subdural space is present, enabling a clear definition of (2) the inner meningeal dural layer and the (4) arachnoid barrier cell membrane. Furthermore, (5) subarachnoid blood vessels, (6) subarachnoid space, (7) trabecular system, (8) brain cortex, and (9) reflection artifacts are depicted by the transdural OCT scan. Red line indicates the area of enlarged excerpt. C Enlarged excerpt demonstrating details of transdural OCT scan. D Schematic drawing of microstructures: (1) + (2) dura mater, (1) outer endosteal layer, (2) inner meningeal layer, (3) subdural space, (4) subarachnoid space (4) arachnoid barrier cell membrane, (5) subarachnoid blood vessels, (6) subarachnoid space, (7) trabecular system, (8) brain cortex, and (9) reflection artifacts; see Hartmann et al. [16], figure edited with permission from the authors. E OCT scan of frontal lobe at frontal operculum visualizing the collapsed SAS after CSF release, with adjacent internal blood vessels. Red rectangle shows enlarged details of the OCT-Scan; see Hartmann et al. [17, 18], figure edited with permission from the authors. F Light microscopic intraoperative image of parent vessel: right internal carotid artery. Orange horizontal line indicates area of OCT scan. G OCT scan of parent vessel. (1 Tunica externa; (2) tunica media; (3) tunica interna; (4) atherosclerotic plaque; (5) vasa vasorum. H Light microscopic intraoperative image of ramus communicans aneurysm seen from a left fronto-lateral approach. I OCT scan of the neck of the ramus communicans anterior aneurysm (CA) demonstrating the continuous fading transition from a 3-layered configuration of the parent vessel to the mono-layered appearance of the CA dome. (1) CA dome; (2) CA neck; (3) parent vessel. J Light microscopic intraoperative image of right proximal internal carotid artery aneurysm seen from a right fronto-lateral approach; orange lines indicate the area of OCT scan at the aneurysm dome with artherosclerotic plaque. K Longitudinal OCT scan at aneurysm dome demonstrating intra-aneurysmatic atherosclerotic plaque; see Hartmann et al. [17, 18], figures edited with permission from the authors
Arachnoid cyst
Microscope-integrated OCT could demonstrate the membrane of a middle fossa cerebral arachnoid cyst. Transcystic OCT at site of the temporal lobe delineated the trabecular system of the arachnoid space, inlying cerebral arteries and veins as well as the brain cortex. At site of fenestration, OCT excluded hidden crucial anatomic structures prior to the dissection of the membrane [16].
Peripheral nerves
Intraoperative handheld OCT during peripheral nerve surgery could delineate single bundles of nerve fascicles [6]. Image quality was influenced by motion artifacts and wrapping of the imaging probe with sterile foil. Microscope-integrated systems would eliminate these restraints and improve the surgical work flow. Clinical relevant data which correlates the rehabilitation potential with intraoperative OCT similar to the work published on optic nerve rehabilitation are missing {Wilson:2020br}.
Conclusion
Intraoperative OCT offers the possibility to study microanatomy in vivo approaching the resolution of conventional histology. Manifold applications could deepen our physiological and pathophysiological understanding; e.g., in case of the choroid plexus, OCT videos could elicit mechanisms of liquor production in correlation to blood pulse.
Data which proves that microscope-integrated OCT lowers the perioperative morbidity or extent of tumor resection does not exist.
Experience from our group suggests that the regular use of microscope-integrated OCT could increase security during certain critical surgical steps. In case of dural dissection during transtentorial approaches, tumor resection at mayor venous blood vessels like sigmoid sinus, removal of craniopharyngiomas, transsulcal preparation, and dissection of the Sylvian fissure - OCT could delineate crucial structures prior to dissection. Here, augmented reality is needed for intuitive integration into the microsurgical workflow.
For microsurgical considerations, it is worth noting that valuable OCT scanning is only possible if the surgical trajectory exposes the pathology in an orthograde scanning angel. This general principle of microscope-integrated OCT is of particular significance during key hole surgery or other narrow approaches, e.g., to ramus communicans anterior aneurysms or supracerebellar infratentorial approaches to the pineal region.
Integration of OCT in endoscopy could aid surgeries with no access for real-time imaging methods like sonography. In case of hypophyseal surgery, OCT might define concealed hypophyseal arteries, cavernous sinus walls, and inlying structures as well as tumor and hypophyseal tissue to extend resection while lowering perioperative morbidity.
Combinations of OCT and minimal invasive needle devices seem to hold promise in tumor surgery. Biopsy needles with integrated forward and lateral viewing probes could lower perioperative morbidity by securing blood vessels and functional relevant brain structures as well as control biopsy positioning. Combination of OCT and laser ablation further offers the possibility to perform “optic biopsies” and adapt the coagulation process in real time. In the emerging field of minimal invasive surgery, these systems might gain further relevance.
OCT offers unprecedented quality to delineate the microstructural composition of vessel walls and their pathologies. In aneurysm surgery, OCT of the neck of the aneurysm could help to aid clip placement in relation to intravascular arteriosclerosis, thrombosis, aneurysm wall thickness, and vessel wall calcifications—characteristics and pathologies which were concealed so far. In case of bypass surgery OCT would be an imaging method which could aid to determine optimal site of bypass in correlation to vessel wall pathologies.
Neuroscientific advancements like diffusion-dependent OCT for functional brain imaging have not yet found clinical applications. Brain pulsation and vessel artifacts as well as intermodality validation with hemodynamic and electrophysiological measurements still inhibit clinical transfer [32].
Spatial resolution of polarization-sensitive OCT lays yet beyond the scope of manual microsurgery. If robotic surgery further develops, OCT might gain novel importance as a real-time distance measuring tool.
Data Availability
Not applicable for a review. All citations in the text and figures are given.
Code Availability
Not applicable for a review.
References
Almasian M, Wilk LS, Bloemen PR, van Leeuwen TG, Ter Laan M, Aalders MCG (2019) Pilot feasibility study of in vivo intraoperative quantitative optical coherence tomography of human brain tissue during glioma resection. J Biophotonics 12:e201900037. https://doi.org/10.1002/jbio.201900037
Assayag O, Grieve K, Devaux B, Harms F, Pallud J, Chretien F, Boccara C, Varlet P (2013) Imaging of non-tumorous and tumorous human brain tissues with full-field optical coherence tomography. YNICL 2:549–557. https://doi.org/10.1016/j.nicl.2013.04.005
Beaurepaire E, Moreaux L, Amblard F, Mertz J (1999) Combined scanning optical coherence and two-photon-excited fluorescence microscopy. Optics Letters 24:969–971. https://doi.org/10.1364/OL.24.000969
Boppart SA (2003) Optical coherence tomography: Technology and applications for neuroimaging. Psychophysiology 40:529–541. https://doi.org/10.1111/1469-8986.00055
Böhringer HJ, Lankenau E, Stellmacher F, Reusche E, Hüttmann G, Giese A (2009) Imaging of human brain tumor tissue by near-infrared laser coherence tomography. Acta Neurochir (Wien) 151:507–517; discussion 517. https://doi.org/10.1007/s00701-009-0248-y
Carolus AE, Lenz M, Hofmann M, Welp H, Schmieder K, Brenke C (2019) High-resolution in vivo imaging of peripheral nerves using optical coherence tomography: a feasibility study. J Neurosurg 132:1907–1913. https://doi.org/10.3171/2019.2.JNS183542
Chang W, Fan Y, Zhang X, Liao H (n.d.) An intelligent theranostics method using optical coherence tomography guided automatic laser ablation for neurosurgery. In: Presented at the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, pp 3224–3227. https://doi.org/10.1109/EMBC.2018.8513016
Chen C-J, Kumar JS, Chen SH, Ding D, Buell TJ, Sur S, Ironside N, Luther E, Ragosta M, Park MS, Kalani MY, Liu KC, Starke RM (2018) Optical coherence tomography: future applications in cerebrovascular imaging. Stroke 49:1044–1050. https://doi.org/10.1161/STROKEAHA.117.019818
Drexler W (2003) Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol 121:695–706. https://doi.org/10.1001/archopht.121.5.695
Ellerbee AK (2014) Optical coherence tomography: technology and applications, in:. Presented at the 2014 IEEE Photonics Conference (IPC), IEEE, pp 7–8. https://doi.org/10.1109/IPCon.2014.6994962
Fan Y, Xia Y, Zhang X, Sun Y, Tang J, Zhang L, Liao H (2018) Optical coherence tomography for precision brain imaging, neurosurgical guidance and minimally invasive theranostics. BST 12:12–23. https://doi.org/10.5582/bst.2017.01258
Fujimoto JG, Pitris C, Boppart SA, Brezinski ME (2000) Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia 2:9–25. https://doi.org/10.1038/sj.neo.7900071
Gagnon L, Smith AF, Boas DA, Devor A, Secomb TW, Sakadžić S (2016) Modeling of cerebral oxygen transport based on in vivo microscopic imaging of microvascular network structure, blood flow, and oxygenation. Front Comput Neurosci 10:82–20. https://doi.org/10.3389/fncom.2016.00082
Giese A, Böhringer HJ, Leppert J, Kantelhardt SR, Lankenau E, Koch P, Birngruber R, Hüttmann G (2006) Non-invasive intraoperative optical coherence tomography of the resection cavity during surgery of intrinsic brain tumors. In: Kollias N, Zeng H, Choi B, Malek RS, Wong BJ, Ilgner JFR, Trowers EA, de Riese WT, Hirschberg H, Madsen SJ, Lucroy MD, Tate LP, Gregory KW, Tearney GJ (Eds) Photonic therapeutics and diagnostics II, SPIE Proceedings. International Society for Optics and Photonics, p 60782Z. https://doi.org/10.1117/12.674436
Hartmann K, Stein K-P, Neyazi B, Sandalcioglu IE (2020) Optical coherence tomography of cranial dura mater: Microstructural visualization in vivo. Clin Neurol Neurosurg 200:106370. https://doi.org/10.1016/j.clineuro.2020.106370
Hartmann K, Stein K-P, Neyazi B, Sandalcioglu IE (2020) Microscope integrated optical coherence tomography of a cerebral arachnoid cyst: a new technique to increase intraoperative security. J Clin Neurosci 82:29–31. https://doi.org/10.1016/j.jocn.2020.10.008
Hartmann K, Stein K-P, Neyazi B, Sandalcioglu IE (2019) First in vivo visualization of the human subarachnoid space and brain cortex via optical coherence tomography. Ther Adv Neurol Disord 12:175628641984304. https://doi.org/10.1177/1756286419843040
Hartmann K, Stein K-P, Neyazi B, Sandalcioglu IE (2019) Aneurysm Architecture: first in vivo imaging of human cerebral aneurysms with extravascular optical coherence tomography. Cerebrovasc Dis 48:1–6. https://doi.org/10.1159/000502450
Izatt J (2012) Intraoperative OCT for Vitreoretinal Surgery, in:. Presented at the Biomedical Optics, OSA, Washington, D.C., p BTu2B.1. https://doi.org/10.1364/BIOMED.2012.BTu2B.1
Kang JU, Huang Y, Zhang K, Ibrahim Z, Cha J, Lee WPA, Brandacher G, Gehlbach PL (2012) Special section on optical diagnostic and biophotonic methods from bench to bedside: real-time three-dimensional Fourier-domain optical coherence tomography video image guided microsurgeries. J Biomed Opt 17:081403–81401. https://doi.org/10.1117/1.JBO.17.8.081403
Katta N, Estrada AD, McElroy AB, Gruslova A, Oglesby M, Cabe AG, Feldman MD, Fleming RD, Brenner AJ, Milner TE (2019) Laser brain cancer surgery in a xenograft model guided by optical coherence tomography. Theranostics 9:3555–3564. https://doi.org/10.7150/thno.31811
Kiseleva EB, Yashin KS, Moiseev AA, Timofeeva LB, Kudelkina VV, Alekseeva AI, Meshkova SV, Polozova AV, Gelikonov GV, Zagaynova EV, Gladkova ND (2019) Optical coefficients as tools for increasing the optical coherence tomography contrast for normal brain visualization and glioblastoma detection. Neurophoton 6:035003. https://doi.org/10.1117/1.NPh.6.3.035003
Kuo W-C, Kao M-C, Tsou M-Y, Ting C-K (2017) In vivo images of the epidural space with two- and three-dimensional optical coherence tomography in a porcine model. PLoS ONE 12:e0172149-e172214. https://doi.org/10.1371/journal.pone.0172149
Kut C, Chaichana KL, Xi J, Raza SM, Ye X, McVeigh ER, Rodriguez FJ, Quiñones-Hinojosa A, Li X (2015) Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci Transl Med 7:292ra100-292ra100. https://doi.org/10.1126/scitranslmed.3010611
Lankenau E, Klinger D, Winter C, Malik A, Müller HH, Oelckers S, Pau H-W, Just T, Hüttmann G (2007) Combining optical coherence tomography (OCT) with an operating microscope In: Advances in Medical Engineering, Springer Proceedings in Physics. Springer, Berlin, Heidelberg, Berlin, Heidelberg, pp 343–348. https://doi.org/10.1007/978-3-540-68764-1_57
Lankenau EM, Krug M, Oelckers S, Schrage N, Just T, Hüttmann G (2013) iOCT with surgical microscopes: a new imaging during microsurgery. Adv Opt Technol 2:233–239. https://doi.org/10.1515/aot-2013-0011
Lee D, Lee C, Kim S, Zhou Q, Kim J, Kim C (2016) In vivo near infrared virtual intraoperative surgical photoacoustic optical coherence tomography. Sci Rep 6:1–10. https://doi.org/10.1038/srep35176
Liang C-P, Wierwille J, Moreira T, Schwartzbauer G, Jafri MS, Tang C-M, Chen Y (2011) A forward-imaging needle-type OCT probe for image guided stereotactic procedures. Opt Express OE 19:26283–26294. https://doi.org/10.1364/OE.19.026283
Liba O, SoRelle ED, Sen D, de la Zerda A (2017) High sensitivity contrast enhanced optical coherence tomography for functional in vivo imaging. In: Fujimoto JG, Izatt JA, Tuchin VV (Eds.) Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine XXI, SPIE Proceedings. International Society for Optics and Photonics, p 100531D. https://doi.org/10.1117/12.2248258
Lopez WOC, Ângelos JS, Martinez RCR, Takimura CK, Teixeira MJ, Lemos Neto PA, Fonoff ET (2015) Optical coherence tomography imaging of the basal ganglia: feasibility and brief review. Braz J Med Biol Res 48:1156–1159. https://doi.org/10.1590/1414-431X20154679
Nakaji H, Kouyama N, Muragaki Y, Kawakami Y, Iseki H (2008) Localization of nerve fiber bundles by polarization-sensitive optical coherence tomography. J Neurosci Methods 174:82–90. https://doi.org/10.1016/j.jneumeth.2008.07.004
Pouratian N, Sheth S, Bookheimer SY, Martin NA, Toga AW (2003) Applications and limitations of perfusion-dependent functional brain mapping for neurosurgical guidance. Neurosurg Focus 15:E2-8. https://doi.org/10.3171/foc.2003.15.1.2
Pouratian N, Sheth SA, Martin NA, Toga AW (2003) Shedding light on brain mapping: advances in human optical imaging. Trends Neurosci 26:277–282. https://doi.org/10.1016/S0166-2236(03)00070-5
Ramakonar H, Quirk BC, Kirk RW, Li J, Jacques A, Lind CRP, McLaughlin RA (2018) Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci Adv 4:eaav4992. https://doi.org/10.1126/sciadv.aav4992
Uga M, Dan I, Sano T, Dan H, D, E.W.M., (2014) Optimizing the general linear model for functional near-infrared spectroscopy: an adaptive hemodynamic response function approach. Neurophoton 1:015004. https://doi.org/10.1117/1.NPh.1.1.015004
Valdés PA, Roberts DW, Lu F-K, Golby A (2016) Optical technologies for intraoperative neurosurgical guidance. Neurosurg Focus 40:E8. https://doi.org/10.3171/2015.12.FOCUS15550
Wang H, Magnain C, Wang R, Dubb J, Varjabedian A, Tirrell LS, Stevens A, Augustinack JC, Konukoglu E, Aganj I, Frosch MP, Schmahmann JD, Fischl B, Boas DA (2018) as-PSOCT: volumetric microscopic imaging of human brain architecture and connectivity. NeuroImage 165:56–68. https://doi.org/10.1016/j.neuroimage.2017.10.012
Wicks RT, Huang Y, Zhang K, Zhao M, Tyler BM, Suk I, Hwang L, Ruzevick J, Jallo G, Brem H, Pradilla G, Kang JU (2014) Extravascular optical coherence tomography. Stroke 45:1123–1130. https://doi.org/10.1161/STROKEAHA.113.002970
Yashin KS, Kiseleva EB, Gubarkova EV, Moiseev AA, Kuznetsov SS, Shilyagin PA, Gelikonov GV, Medyanik IA, Kravets LY, Potapov AA, Zagaynova EV, Gladkova ND (2019) Cross-polarization optical coherence tomography for brain tumor imaging. Front Oncol 9:201. https://doi.org/10.3389/fonc.2019.00201
Yashin KS, Kiseleva EB, Moiseev AA, Kuznetsov SS, Timofeeva LB, Pavlova NP, Gelikonov GV, Medyanik IA, Kravets LY, Zagaynova EV, Gladkova ND (2019) Quantitative nontumorous and tumorous human brain tissue assessment using microstructural co- and cross-polarized optical coherence tomography. Sci Rep 9:2024. https://doi.org/10.1038/s41598-019-38493-y
Yecies D, Liba O, Zerda AdL, Grant GA (2018) Intraoperative Imaging Modalities and the Potential Role of Speckle Modulating Optical Coherence Tomography. Neurosurgery 65:74–77. https://doi.org/10.1093/neuros/nyy199
Zammar SG, Hamade YJ, El Tecle NE, El Ahmadieh TY, Caughel KCM, Carroll TJ, Bendok BR (2014) Optical coherence tomography as a potential tool for extravascular imaging in vascular neurosurgery. Neurosurgery 75:N17-8. https://doi.org/10.1227/01.neu.0000452315.99484.41
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Karl Hartmann: Conceptualization, methodology, validation, formal analysis, data curation, visualization, sketching, writing, editing. Klaus-Peter Stein: Conceptualization, reviewing, and editing. Belal Neyazi: Conceptualization, investigation. I. Erol Sandalcioglu: Validation, reviewing, editing, supervision.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the studies of our research group involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The studies of our research group were further approved by the local ethics committee (No. 3012–2016).
Informed consent
Informed consent was obtained from all individual participants included in the studies of our research group.
Consent for publication
Consent for publication was obtained from all individual participants included in studies of our research group.
Conflict of interest
The authors declare no competing interests. OptoMedical Technologies GmbH supported our research group with free equipment for iOCT.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hartmann, K., Stein, KP., Neyazi, B. et al. Theranostic applications of optical coherence tomography in neurosurgery?. Neurosurg Rev 45, 421–427 (2022). https://doi.org/10.1007/s10143-021-01599-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-021-01599-x