Abstract
Intraoperative MRI (ioMRI) has become a frequently used tool to improve maximum safe resection in brain tumor surgery. The usability of intraoperatively acquired diffusion-weighted imaging sequences to predict the extent and clinical relevance of new infarcts has not yet been studied. Furthermore, the question of whether more aggressive surgery after ioMRI leads to more or larger infarcts is of crucial interest for the surgeons’ operative strategy. Retrospective single-center analysis of a prospective registry of procedures from 2013 to 2019 with ioMRI was used. Infarct volumes in ioMRI/poMRI, lesion localization, mRS, and NIHSS were analyzed for each case. A total of 177 individual operations (60% male, mean age 45.5 years old) met the inclusion criteria. In 61% of the procedures, additional resection was performed after ioMRI, which resulted in a significantly higher number of new ischemic lesions postoperatively (p < .001). The development of new or enlarged ischemic areas upon additional resection could also be shown volumetrically (mean volume in ioMRI 0.39 cm3 vs. poMRI 2.97 cm3; p < .001). Despite the surgically induced new infarcts, mRS and NIHSS did not worsen significantly in cases with additional resection. Additionally, new perilesional ischemia in eloquently located tumors was not associated with an impaired neurological outcome. Additional resection after ioMRI leads to new or enlarged ischemic areas. However, these new infarcts do not necessarily result in an impaired neurological outcome, even when in eloquent brain areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraoperative MRI during cranial tumor surgery has become a supplementary standard of care in many neurosurgical institutions for a variety of neurosurgical procedures [4, 12, 22, 23]. The intraoperative images are of great value to provide the surgeon with an updated dataset for navigation, along with intraoperative resection control and early screening for complications such as ischemic complications. Several studies have reported the utility of intraoperative MRI (ioMRI) to maximize safe resection, which, in turn, is linked to prolonged overall survival in patients with, for example, low- and high-grade gliomas [7, 13, 22, 24]. Increasing experience has led to more sophisticated protocols and guidelines, which have improved the safety [1, 19] and the robustness and quality of ioMRI sequences. This accounts not only for routinely used methods but also for advanced imaging methods such as diffusion-weighted imaging (DWI) [17, 25]. DWI sequences acquired during surgery are used as an important tool for the early detection of ischemic brain injuries that might cause new neurological deficits for the patient. Therefore, these sequences potentially influence the surgeon’s decision to continue the resection or not.
Despite the evident advantages of ioMRI, little is known about the influence of DWI sequences on surgical strategy and aggressiveness in terms of resection control and their implications for potential new postoperative deficits. The aim of this study was to characterize the presence and extent of new ischemic lesions in ioMRI. Additionally, we were interested in the question of whether a continuation of resection after intraoperative resection control with MRI might lead to more or enlarged postoperative infarcts.
Methods
Patient selection
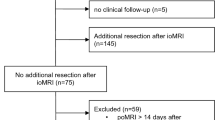
All consecutive patients who underwent neurosurgical procedures using ioMRI with acquired DWI sequences between 01/2013 and 01/2019 at our department were included. Transsphenoidal procedures with ioMRI were excluded because the ioMRI protocol changed during the observation period, and acquisition of DWI sequences in these procedures was omitted; ioMRI was only used inconsistently. Regularly, patients in which resection was extended after ioMRI received postoperative MRI (poMRI) within 72 h. However, in singular cases, no early poMRI was done. To ensure comparability of new DWI restrictions, cases in which surgical resection was expanded after ioMRI poMRI was not conducted within 14 days after surgery were excluded (Fig. 1).
Intraoperative MRI
Our institution uses a 2-room intraoperative MRI suite concept with a 3 Tesla (T) high-field MRI (Siemens 3 T Skyra VD13, Siemens Healthineers, Erlangen, Germany) and a NORAS 8 channel head coil (NORAS MRI products GmbH, Hoechberg, Germany). The safety and quality of intraoperatively acquired MRI sequences were ensured by using a previously published institutional default ioMRI checklist [25].
Data acquisition
Data points were extracted from our departmental prospectively recorded patient registry, which includes a standardized dataset for all neurosurgical procedures since 2013 [20]. The registry was approved by the local ethical review board (“Kantonale Ethikkommission Zürich,” identifier PB-2017-00093), registered at clinicaltrials.gov (NCT01628406), and individual patients’ consent was waived. All data were collected by neurosurgeons at hospital admission and discharge. At discharge, all data entries were validated by the respective surgeon. The modified Rankin Scale [26] (mRS) is used as a general performance scale, and the National Institute of Health Stroke Scale [11] (NIHSS) is used as a neurological outcome scale. For every new team member, it is obligatory to complete an online introductory teaching course and exam to ensure the correct usage of the clinical scores. Missing data for any timepoint were added by retrospective chart review.
Infarct determination and volumetric analysis
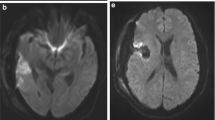
Infarcts were determined using DWI and apparent diffusion coefficient (ADC) image series for each case. Susceptibility weighted imaging sequences were used to rule out artificial DWI restrictions caused by microhemorrhage, especially at the resection cavity. Based on the extent and localization of the DWI restriction, infarcts were grouped into 4 classes in ascending order: 1. point-shaped infarcts, 2. band-shaped infarcts at the resection cavity, 3. sector-shaped infarcts that involve deeper parts of the parenchyma, and 4. territorial infarcts (Fig. 2). If more than one infarct class was present, only the highest class was registered, whereas for volumetric measurements, all infarct areas were summed. Volumetric analysis of infarct volumes from DWI sequences was performed using iPlan Net® (Brainlab AG, Munich, Germany). The relative change in infarct volume was calculated by subtracting intraoperative from postoperative infarct volumes.
Examples of different infarct categories with their corresponding ADC and DWI images. 1. Point-shaped infarct; 2. thin band-shaped infarct at resection cavity; 3. sector-shaped infarct with involvement of deeper parts of the parenchyma; 4. territorial infarct. ADC apparent diffusion coefficient, DWI diffusion-weighted imaging
Statistical analysis
All data processing steps and statistical analyses were performed using R Studio (Version 1.1.463, R Studio Inc.) with open-source libraries. p values < .05 were considered statistically significant. The statistical tests used are indicated in the figure captions or the main text. There were no missing data for the variables included in the analysis.
Results
Clinical characteristics
The inclusion criteria resulted in a cohort of 177 surgical procedures (Fig. 1) in 155 individual patients. The mean age was 45.5 years (± 16.8 SD; 3–76), and 60% of the cases involved male patients. The mean age of patients did not differ significantly between patients with and without new infarcts on poMRI (Mann-Whitney U test, p = .29, Table 1). Sixty percent of the cases were first operations, whereas the remaining cases involved a previous operation on the lesion (Table 1). This difference was not associated with a higher number of new infarcts on poMRI (Pearson’s chi-squared test, p = .2, Table 1). Histopathological entities are listed in Table 1, with WHO grade III (n = 57) and IV (n = 51) gliomas (total n = 145) being the most frequent. Metastases (n = 9) derived most frequently from lung carcinoma (n = 6, Online Resource 1 – Suppl. Table 1). Other histopathological entities included epidermoid cysts (n = 3), central neurocytoma (n = 2), schwannoma (n = 2), and others (Online Resource 1 – Suppl. Table 1).
Determinants of new DWI lesions on poMRI
The frequency of new postoperative infarcts differed between histological tumor entities: gliomas and metastases showed new infarcts in 65% and 56%, respectively, compared with only 30% for other entities (Fisher’s exact test, p = .006, Table 1). WHO classification, however, did not seem to be associated with new infarcts in poMRI (Fisher’s exact test, p = .6, Table 1). Looking at the localization of the operated lesions within the brain, the frontal lobe was the most frequently affected lobe (36%), followed by the perirolandic region (12%) and the temporal lobe (10%). However, the occurrence of new postoperative ischemic lesions was not linked to a distinct anatomical localization of the resected lesion (Fisher’s exact test, p = .13). Intraoperative neuromonitoring (ioNM) was used in 60.5% of resections and declined at least at one point during surgery in 21.5%. Resection was stopped according to operative report because of ioNM worsening in 17% of the cases with ioNM. However, usage, decline, and stop because of decline were not associated with new infarcts in poMRI (Online Resource 1 – Suppl. Table 2).
In 61% (n = 108) of the cases, surgical resection of the lesion was continued after ioMRI. This resulted in a significantly higher number of postoperative ischemic lesions compared with cases without additional resection after ioMRI (Pearson’s chi-squared test, p < .001). Nonetheless, in cases without additional resection, the absence of DWI lesions in ioMRI vs. poMRI showed a specificity of 78.4%. Across the whole study group, the presence of an intraoperative DWI restriction could be confirmed on poMRI in the majority of cases (83.7%, positive predictive value).
Infarct characteristics and quantification
Classifying the infarcts regarding their shape (Fig. 2), the distribution of infarct volumes among the different classes is shown in Fig. 3a for ioMRI and poMRI. Median infarct volumes increased throughout the different infarct classes, and there was an increased mean infarct volume in poMRI compared with ioMRI (Fig. 3a): the increase was slight in band-shaped (1.97 ± 1.81 vs. 1.39 ± 1.32 cm3) and more pronounced in sector-shaped (4.01 ± 4.32 vs. 2.89 ± 2.0 cm3) and territorial infarctions (23.01 ± 19.8 vs. 9.12 cm3). However, statistical comparison (Wilcoxon signed-rank test) showed no significant difference between volumes of different infarct classes in intra- vs. poMRI. Figure 3b shows the absolute numbers of the different infarct classes for ioMRI and poMRI. Fewer cases had no infarct demarcation (n = 67) in poMRI than in ioMRI (n = 134). A total of 94% (n = 63) of cases with new infarct on poMRI underwent additional resection after ioMRI (vs. 6%, n = 4 without additional resection). This increased number of new postoperative infarcts resulted mainly from new band-shaped and sector-shaped infarcts adjacent to the resection cavity, whereas point-shaped and territorial ischemic lesions showed comparable numbers intra- and postoperatively (Fig. 3b).
a Volumetric and morphological characteristics of the new infarcts. Infarct volumes in cm3 of the different infarct categories (Fig. 1) for intraoperative and postoperative MRI (Wilcoxon signed-rank test p > .5 for each infarct class). b Number of demarcated new infarcts of each morphological infarct category (Fig. 1) in intraoperative (red) and postoperative (blue) MRI
Additional resection upon ioMRI
In our case series, a significantly increased overall infarct volume occurred in cases with continuation of resection after ioMRI (Pearson’s chi-squared test, p < .001).
Comparing the infarct volumes regarding additional resection after ioMRI, the cases with additional resection showed significantly increased postoperative infarct volumes (0.39 cm3 vs. 2.97 cm3 mean volume; Fig. 4a left plot, Wilcoxon signed-rank test, p < .001). When comparing the relative changes in infarct volumes between ioMRI and poMRI (Δ infarct volume, Fig. 4b), the enlarged ischemic lesions in cases with additional resection become more evident (Mann-Whitney U test, p < .001). In contrast, in cases where resection was finished after ioMRI, the absolute and relative ischemic volumes did not increase significantly (Fig. 4a right plot, b), although singular cases showed a slightly increased infarct volume in poMRI. The majority of increased infarct volumes resulted from entirely new infarcts in poMRI and less from enlarged or evolved preexisting ones (see Online Resource 2 – Suppl. Fig. 1 and Suppl. Fig. 2 for an illustrative example).
a Comparison of absolute infarct volumes (cm3) in intraoperative (red) and postoperative (blue) MRI (Wilcoxon signed-rank test) for cases with (left plot) and without additional resection (right plot) after intraoperative MRI. b Comparison of relative infarct volume change (postoperative–intraoperative MRI) of cases with (red) and without (blue) additional resection upon intraoperative MRI (Mann-Whitney U test). Δ delta, ns not significant
Moreover, the occurrence of a DWI lesion in ioMRI did not affect the proportion of continued resections (65.1% of cases with new DWI compared with 59.7% without new DWI restriction in ioMRI; Pearson’s chi-squared test p = .65), and the histopathological subdivision was similarly not associated with an altered proportion of continued resection after ioMRI (Fisher’s exact test, p = .08).
Neurological and functional outcome upon new infarct demarcation
We compared the relative changes in NIHSS (Fig. 5a) and mRS (Fig. 5b) at discharge compared with admission for all cases with new infarction on poMRI. The score changes for cases with additional resection did not differ significantly from the cases without additional resection after ioMRI (Mann-Whitney U test, p > .05 for ΔNIHSS and ΔmRS). Looking at the percentages of cases with improved, unchanged, and worsened NIHSS after surgery, there was no significant difference between the group with and the group without new postoperative infarct (Pearson’s chi-squared test p = .7, Fig. 5c).
a, b Comparison of relative changes in NIHSS (a) and mRS (b) at discharge compared with admission for cases with (red) and without (blue) additional resection after intraoperative MRI; only cases with a new infarction on postoperative MRI are shown. c Stacked bar plots indicating the percentages of improved (green), unchanged (blue), or worsened NIHSS scores postoperatively for cases with (upper bar) and without (lower bar) new infarcts. ΔNIHSS delta National Institute of Health Stroke Scale, ΔmRS delta modified Rankin Scale, ns not significant
Relevance of anatomic lesion localization for clinical impact of new infarcts
As new infarcts might only become clinically apparent if they are localized in certain brain areas, we identified the anatomical localization of the operated lesions and their lateralization (Table 1): Resections within the frontal lobe represented the main part of the patient population, followed by lesions involving the perirolandic region. Although there were several resections in the perirolandic region (12%, n = 22), which is considered a highly eloquent brain area, we detected no statistically significant difference in relative NIHSS change between cases with new ischemic areas and those without new infarcts (Table 1 and Online Resource 2 – Suppl. Fig. 3).
Discussion
Intraoperative MRI has become a widely implemented tool in neurosurgical procedures to maximize safe resection for applications such as brain tumor surgery [4, 7, 13, 22]. Despite the improved quality of the intraoperatively acquired MRI sequences [17], little is known about the usability of intraoperatively acquired DWI sequences, their influence on surgical strategy, and the risk and clinical impact of new postoperative infarcts due to an additional resection after ioMRI.
In the current study, we showed that intraoperatively acquired DWI images with a 3T high-field MRI are of enough quality to early rule out or delineate new ischemic lesions.
The usage strategy of ioMRI remains inconsistent, as some consider it as a confirmation of complete tumor removal and resection should be considered finished by the surgeon before ioMRI is performed, others referring to the so called “staged volume” surgery, allowing a stepwise tumor resection by repeating ioMRI and thus increasing safety [9]. However, in many published series, additional resection is performed after acquisition of ioMRI [21], as was also the case in our study population.
Our data clearly show that an additional resection after ioMRI leads to an increased occurrence and volume of ischemic lesions on poMRI. These findings confirmed our expectations since resection after ioMRI is usually continued deeper and often more difficult to access or eloquently seated tumor remnants.
In the histopathological subgroup of gliomas (82% of the cases), the occurrence of new infarcts after additional resection was significantly higher than in other entities. Since the overall goal of maximum (safe) resection is of crucial interest for the long-term outcome of glioma surgery [13, 22, 24], this finding did not surprise us, as usage of intraoperative resection control might be prone to a more aggressive resection compared with cases without.
The differentiation between the first operation or reoperation on the respective cerebral lesion showed no differences regarding the occurrence of new infarcts. This might seem counterintuitive and in contrast with previous findings, which indicate that postoperative ischemic lesions occurred more frequently in recurrent glioma surgery compared with resections of primary gliomas [3]. However, the literature reports remain ambiguous, as another study concluded that recurrent glioma surgery was not associated with more infarcts [2]. In contrast to the cited studies, our patient cohort consists of a broader variety of histopathological entities and not all tumor reoperations might have received radiotherapy and developed postradiogenic tissue changes, which could potentially contribute to a higher rate of infarctions in recurrent surgery [3, 27]. Additionally, the proper use of ioMRI in recurrent glioma surgery might improve safe resection compared with recurrent surgeries without intraoperative image guidance.
To avoid vascular lesions, a combination of central tumor debulking using e.g. ultrasonic aspiration to provide sufficient working space and subsequent sub-pial resection to protect vasculature and normal brain tissue might be recommendable.
Previous studies indicated that increased peritumoral DWI restriction in poMRI is associated with new deficits in patients who underwent glioma surgery [3, 6]. However, our data indicate that new DWI restrictions due to extended resection after ioMRI are not necessarily clinically relevant in terms of impaired neurological outcome. As the current study did not look at the clinical relevance of general new infarcts after surgery but rather that of new infarcts caused by additional resection after ioMRI, our findings do not necessarily contradict the results of previous studies.
In our case series, we observed a few cases where no additional resection was performed after ioMRI but enlarged DWI restrictions were nevertheless detected on poMRI. Although DWI images were previously described to reach a high sensitivity and specificity to detect ischemic brain lesions [5], false-negative reports of DWI sequences are present. This phenomenon of false-negative or underestimated infarcts on MRI is not well described and poorly understood, especially in ioMRI [10, 15, 18] and also in hyperacute stroke imaging [8, 14,15,16]. Possible explanations might be that ioMRI occurs too early to detect hyperacute infarcts or that vasospasms contribute to delayed infarct development without further tissue manipulation by the surgeon. Further studies are necessary to evaluate the role of false-negative MRI with no DWI signal alteration in an intraoperative application and in general.
Conclusion
The surgeon’s decision to continue resection after ioMRI bears the risk of additional and enlarged ischemic brain lesions. However, this study shows that these new infarcts do not necessarily result in an impaired neurological outcome, even if the operation occurs in eloquent brain areas.
Data availability
From the corresponding author upon reasonable request.
References
Dinevski N, Sarnthein J, Vasella F, Fierstra J, Pangalu A, Holzmann D, Regli L, Bozinov O (2017) Postoperative neurosurgical infection rates after shared-resource intraoperative magnetic resonance imaging: a single-center experience with 195 cases. World Neurosurg 103:275–282. https://doi.org/10.1016/j.wneu.2017.03.093
Dutzmann S, Gessler F, Bink A, Quick J, Franz K, Seifert V, Senft C (2012) Risk of ischemia in glioma surgery: comparison of first and repeat procedures. J Neuro-Oncol 107:599–607. https://doi.org/10.1007/s11060-011-0784-1
Gempt J, Forschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, Zimmer C, Meyer B, Ringel F (2013) Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg 118:801–808. https://doi.org/10.3171/2012.12.JNS12125
Ginat DT, Swearingen B, Curry W, Cahill D, Madsen J, Schaefer PW (2014) 3 Tesla intraoperative MRI for brain tumor surgery. J Magn Reson Imaging 39:1357–1365
Gonzalez RG, Schaefer PW, Buonanno FS, Schwamm LH, Budzik RF, Rordorf G, Wang B, Sorensen AG, Koroshetz WJ (1999) Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 210:155–162. https://doi.org/10.1148/radiology.210.1.r99ja02155
Jakola AS, Berntsen EM, Christensen P, Gulati S, Unsgard G, Kvistad KA, Solheim O (2014) Surgically acquired deficits and diffusion weighted MRI changes after glioma resection--a matched case-control study with blinded neuroradiological assessment. PLoS One 9:e101805. https://doi.org/10.1371/journal.pone.0101805
Knauth M, Wirtz CR, Tronnier VM, Aras N, Kunze S, Sartor K (1999) Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol 20:1642–1646
Lefkowitz D, LaBenz M, Nudo SR, Steg RE, Bertoni JM (1999) Hyperacute ischemic stroke missed by diffusion-weighted imaging. AJNR Am J Neuroradiol 20:1871–1875
Leroy HA, Delmaire C, Le Rhun E, Drumez E, Lejeune JP, Reyns N (2019) High-field intraoperative MRI and glioma surgery: results after the first 100 consecutive patients. Acta Neurochir 161:1467–1474. https://doi.org/10.1007/s00701-019-03920-6
Masuda Y, Akutsu H, Ishikawa E, Matsuda M, Masumoto T, Hiyama T, Yamamoto T, Kohzuki H, Takano S, Matsumura A (2018) Evaluation of the extent of resection and detection of ischemic lesions with intraoperative MRI in glioma surgery: is intraoperative MRI superior to early postoperative MRI? J Neurosurg 131:209–216. https://doi.org/10.3171/2018.3.JNS172516
Muir KW, Weir CJ, Murray GD, Povey C, Lees KR (1996) Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 27:1817–1820. https://doi.org/10.1161/01.str.27.10.1817
Muscas G, Bas van Niftrik CH, Fierstra J, Piccirelli M, Sebok M, Burkhardt JK, Valavanis A, Pangalu A, Regli L, Bozinov O (2019) Feasibility and safety of intraoperative BOLD functional MRI cerebrovascular reactivity to evaluate extracranial-to-intracranial bypass efficacy. Neurosurg Focus 46:E7. https://doi.org/10.3171/2018.11.FOCUS18502
Neidert MC, Hostettler IC, Burkhardt JK, Mohme M, Held U, Kofmehl R, Eisele G, Woernle CM, Regli L, Bozinov O (2016) The influence of intraoperative resection control modalities on survival following gross total resection of glioblastoma. Neurosurg Rev 39:401–409. https://doi.org/10.1007/s10143-015-0698-z
Oppenheim C, Stanescu R, Dormont D, Crozier S, Marro B, Samson Y, Rancurel G, Marsault C (2000) False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 21:1434–1440
Prabhu SS, Levine NB, Rao G, Shah K, Weinberg J (2009) Report of negative diffusion-weighted MR imaging during tumor resections using intraoperative MRI. Stereotact Funct Neurosurg 87:304–308. https://doi.org/10.1159/000230693
Rathakrishnan R, Sharma VK, Chan BP (2008) Diffusion-negative MRI in acute ischemic stroke: a case report. Cases J 1:65. https://doi.org/10.1186/1757-1626-1-65
Roder C, Haas P, Tatagiba M, Ernemann U, Bender B (2019) Technical limitations and pitfalls of diffusion-weighted imaging in intraoperative high-field MRI. Neurosurg Rev. https://doi.org/10.1007/s10143-019-01206-0
Saint-Martin C, Apuzzo S, Salman A, Farmer JP (2019) Hyperacute infarct on intraoperative diffusion imaging of pediatric brain tumor surgery. Can J Neurol Sci 46:550–558. https://doi.org/10.1017/cjn.2019.226
Sarnthein J, Luchinger R, Piccirelli M, Regli L, Bozinov O (2016) Prevalence of complications in intraoperative magnetic resonance imaging combined with neurophysiologic monitoring. World Neurosurg 93:168–174. https://doi.org/10.1016/j.wneu.2016.05.097
Sarnthein J, Stieglitz L, Clavien PA, Regli L (2016) A patient registry to improve patient safety: recording general neurosurgery complications. PLoS One 11:e0163154. https://doi.org/10.1371/journal.pone.0163154
Scherer M, Jungk C, Younsi A, Kickingereder P, Muller S, Unterberg A (2016) Factors triggering an additional resection and determining residual tumor volume on intraoperative MRI: analysis from a prospective single-center registry of supratentorial gliomas. Neurosurg Focus 40:E4. https://doi.org/10.3171/2015.11.FOCUS15542
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003. https://doi.org/10.1016/s1470-2045(11)70196-6
Serra C, Burkhardt JK, Esposito G, Bozinov O, Pangalu A, Valavanis A, Holzmann D, Schmid C, Regli L (2016) Pituitary surgery and volumetric assessment of extent of resection: a paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg Focus 40:E17. https://doi.org/10.3171/2015.12.FOCUS15564
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Stienen MN, Fierstra J, Pangalu A, Regli L, Bozinov O (2019) The Zurich checklist for safety in the intraoperative magnetic resonance imaging suite: technical note. Oper Neurosurg (Hagerstown) 16:756–765. https://doi.org/10.1093/ons/opy205
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607. https://doi.org/10.1161/01.str.19.5.604
Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, Kleinberg L (2014) Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol 10:1277–1297. https://doi.org/10.2217/fon.13.271
Funding
Open access funding provided by University of Zurich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
“Kantonale Ethikkommission Zürich,” identifier PB-2017-00093
Consent to participate
Individual patients’ consent was waived.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voglis, S., Müller, T., van Niftrik, C.H.B. et al. Impact of additional resection on new ischemic lesions and their clinical relevance after intraoperative 3 Tesla MRI in neuro-oncological surgery. Neurosurg Rev 44, 2219–2227 (2021). https://doi.org/10.1007/s10143-020-01399-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-020-01399-9