Abstract
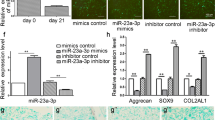
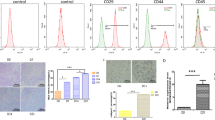
Improved chondrogenic differentiation of mesenchymal stem cells (MSCs) by genetic regulation is a potential method for regenerating articular cartilage. LncRNA MIR22HG has been proven to accelerate osteogenic differentiation, but the regulation mechanism of chondrogenic differentiation is still unclear. Human adipose-derived stem cells (hADSCs) have been widely utilised for bone tissue engineering applications. The present study aimed to examine the effect of MIR22HG on the chondrogenic differentiation of hADSCs. The results confirmed that MIR22HG was downregulated in the process of chondrogenic differentiation. Subsequently, gain- and loss-of-function of MIR22HG experiments showed that the overexpression of MIR22HG suppressed the deposition of cartilage matrix proteoglycans and decreased the expression of cartilage-related markers (e.g. Sox9, ACAN and Col2A1), whereas the knockdown of MIR22HG had the opposite effect. MIR22HG could bind to CTCF (CCCTC-binding factor), and CTCF could bind to the CRLF1 (cytokine receptor-like factor 1) promoter and upregulate CRLF1 gene expression. Besides, inhibition of CRLF1 can reverse the effect of MIR22HG on cell chondrogenic differentiation of hADSCs. Taken together, our outcomes reveal that MIR22HG suppressed chondrogenic differentiation by interaction with CTCF to stabilise CRLF1.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article.
References
Dey BK, Mueller AC, Dutta A (2014) Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 5(4):e944014. https://doi.org/10.4161/21541272.2014.944014
Feng L, Yang Z, Li Y, Wang H, Lo J, Zhang X et al (2021) Linc-ROR promotes mesenchymal stem cells chondrogenesis and cartilage formation via regulating SOX9 expression. Osteoarthr Cartil 29(4):568–578. https://doi.org/10.1016/j.joca.2020.12.020
Franco MM, Prickett AR, Oakey RJ (2014) The role of CCCTC-binding factor (CTCF) in genomic imprinting, development, and reproduction. Biol Reprod 91(5):125. https://doi.org/10.1095/biolreprod.114.122945
Geisler S, Coller J (2013) RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol 14(11):699–712. https://doi.org/10.1038/nrm3679
Gugjoo MB, Sharma GT, Aithal HP, Kinjavdekar P (2016) Cartilage tissue engineering: role of mesenchymal stem cells along with growth factors & scaffolds. Indian J Med Res 144(3):339–347. https://doi.org/10.4103/0971-5916.198724
Jin C, Jia L, Tang Z, Zheng Y (2020a) Long non-coding RNA MIR22HG promotes osteogenic differentiation of bone marrow mesenchymal stem cells via PTEN/ AKT pathway. Cell Death Dis 11(7):601. https://doi.org/10.1038/s41419-020-02813-2
Krych AJ, Saris DBF, Stuart MJ (2020) Cartilage injury in the knee: assessment and treatment options. 28(22):914–922. https://doi.org/10.5435/jaaos-d-20-00266
Lee SH, Kim YJ, Kim YH, Kim HY, Bhang SH (2022) Enhancing therapeutic efficacy of human adipose-derived stem cells by modulating photoreceptor expression for advanced wound healing. 13(1):215. https://doi.org/10.1186/s13287-022-02892-2
Li R, Li B, Cao Y, Li W, Dai W, Zhang L et al (2021) Mir22hg long non-coding RNA -derived miR-22-3p promotes skeletal muscle differentiation and regeneration by inhibiting HDAC4. Mol Ther Nucleic Acids 24:200–211. https://doi.org/10.1016/j.omtn.2021.02.025
Liao S, Meng H, Li J, Zhao J, Xu Y, Wang A et al (2021) Potential and recent advances of microcarriers in repairing cartilage defects. J Orthop Translat 27:101–109. https://doi.org/10.1016/j.jot.2020.10.005
Liu F, Song DY, Huang J, Yang HQ, You D, Ni JD (2021b) Long non-coding RNA CIR inhibits chondrogenic differentiation of mesenchymal stem cells by epigenetically suppressing ATOH8 via methyltransferase EZH2. 27(1):12. https://doi.org/10.1186/s10020-021-00272-9
Liu Y, Shah KM, Luo J (2021a) Strategies for articular cartilage repair and regeneration. Front Bioeng Biotechnol 9:770655. https://doi.org/10.3389/fbioe.2021.770655
Long H, Li Q, Xiao Z, Yang B (2021) LncRNA MIR22HG promotes osteoarthritis progression via regulating miR-9-3p/ADAMTS5 pathway. Bioengineered 12(1):3148–3158. https://doi.org/10.1080/21655979.2021.1945362
Razmara E, Bitaraf A, Yousefi H, Nguyen T, Garshasbi M, Cho W et al (2019) Non-coding RNAs in cartilage development: an updated review. Int J Mol Sci 20(18). https://doi.org/10.3390/ijms20184475
Sur S, Steele R, Ko BCB, Zhang J, Ray RB (2022) Long noncoding RNA ELDR promotes cell cycle progression in normal oral keratinocytes through induction of a CTCF-FOXM1-AURKA signaling axis. J Biol Chem 298(5):101895. https://doi.org/10.1016/j.jbc.2022.101895
Wang F, Tang Z, Shao H, Guo J, Tan T, Dong Y et al (2018) Long noncoding RNA HOTTIP cooperates with CCCTC-binding factor to coordinate HOXA gene expression. Biochem Biophys Res Commun 500(4):852–859. https://doi.org/10.1016/j.bbrc.2018.04.173
Wei W, Dai H (2021) Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioact Mater 6(12):4830–4855. https://doi.org/10.1016/j.bioactmat.2021.05.011
Xu H, Ding C, Guo C, Xiang S, Wang Y, Luo B et al (2021) Suppression of CRLF1 promotes the chondrogenic differentiation of bone marrow-derived mesenchymal stem and protects cartilage tissue from damage in osteoarthritis via activation of miR-320. 27(1):116. https://doi.org/10.1186/s10020-021-00369-1
Yang Q, Guo J, Ren Z, Li B, Huang H, Yang Z (2021b) LncRNA NONHSAT030515 promotes the chondrogenic differentiation of human adipose-derived stem cells via regulating the miR-490-5p/BMPR2 axis. 16(1):658. https://doi.org/10.1186/s13018-021-02757-z
Yang Z, Ren Z, She R, Ao J, Wa Q, Sun Z et al (2021a) miR-23a-3p regulated by LncRNA SNHG5 suppresses the chondrogenic differentiation of human adipose-derived stem cells via targeting SOX6/SOX5. Cell Tissue Res 383(2):723–733. https://doi.org/10.1007/s00441-020-03289-4
Zhao BM, Cheng FH, Cai L (2019) Long noncoding RNA AFAP1-AS1 promoted osteosarcoma proliferation and invasion via upregulating BDNF. Eur Rev Med Pharmacol Sci 23(7):2744–2749. https://doi.org/10.26355/eurrev_201904_17547
Zhu J, Fu Q, Shao J, Peng J, Qian Q, Zhou Y et al (2021a) Over-expression of MEG3 promotes differentiation of bone marrow mesenchymal stem cells into chondrocytes by regulating miR-129-5p/RUNX1 axis. Cell Cycle 20(1):96–111. https://doi.org/10.1080/15384101.2020.1863043
Zhu Y, Li R, Wen L (2021b) Long non-coding RNA XIST regulates chondrogenic differentiation of synovium-derived mesenchymal stem cells from temporomandibular joint via miR-27b-3p/ADAMTS-5 axis. Cytokine 137:155352. https://doi.org/10.1016/j.cyto.2020.155352
Acknowledgements
We thank all members of the laboratory for their kindness and help.
Funding
This work was supported by National Natural Science Foundation of China (31660265 and 31960208), Youth Fund of Guizhou Provincial People’s Hospital (GZSYQN(2015)06), Subsidy Foundation of National Natural Science Foundation of Guizhou Provincial People’s Hospital (Guizhou Science and Technology Platform (2017)5724), Science and Technology Foundation of Guizhou Province (Guizhou Science and Technology J Word (2015)2096) and Subsidy Foundation of National Natural Science Foundation of Guizhou Provincial People’s Hospital (GPPH-NSFC-2019-1).
Author information
Authors and Affiliations
Contributions
J.G., Z.R., and Z.Y. designed and performed the research; J.G. and W.Y. data collection; X.W., H.H., and B.L. data analysis and interpretation; J.G. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and informed consent
HADSCs were collected from healthy people by liposuction aspiration through the ethical standards of the Ethics Committee of Guizhou Provincial People’s Hospital. Written informed consent was received from the donors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, J., Ye, W., Wu, X. et al. Long non-coding RNA MIR22HG suppresses the chondrogenic differentiation of human adipose-derived stem cells by interacting with CTCF to upregulate CRLF1. Funct Integr Genomics 23, 329 (2023). https://doi.org/10.1007/s10142-023-01248-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01248-0