Abstract
Purpose
To assess the healing of costal cartilage fractures (CCFX) in patients with blunt polytrauma with follow-up imaging and clinical examination. Effect on physical performance and quality of life (QoL) was also evaluated.
Methods
The study group comprised twenty-one patients with diagnosed CCFX in trauma CT. All the patients underwent MRI, ultrasound, ultra-low-dose CT examinations, and clinical status control. The patients completed QoL questionnaires. Two radiologists evaluated the images regarding fracture union, dislocation, calcifications, and persistent edema at fracture site. An attending trauma surgeon clinically examined the patients, with emphasis on focal tenderness and ribcage mobility. Trauma registry data were accessed to evaluate injury severity and outcome.
Results
The patients were imaged at an average of 34.1 months (median 36, range 15.8–57.7) after the initial trauma. In 15 patients (71.4%), CCFX were considered stable on imaging. Cartilage calcifications were seen on healed fracture sites in all the patients. The fracture dislocation had increased in 5 patients (23.8%), and 1 patient (4.8%) showed signs of a non-stable union. Four patients (19.0%) reported persistent symptoms from CCFX.
Conclusion
Non-union in CCFX is uncommon but may lead to decreased stability and discomfort. Both clinical and radiological examinations play an important part in the post-traumatic evaluation of CCFX. CT and MRI visualize the healing process, while dynamic ultrasound may reveal instability. No significant difference in QoL was detected between patients with radiologically healed and non-healed CCFX. Post-traumatic disability was mostly due to other non-thoracic injuries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blunt chest trauma is common and a major cause of morbidity in hospitalized patients [1,2,3]. The most common mechanisms of blunt thoracic injury are motor vehicle collisions, motorcycle accidents, and falls. Although most patients with blunt chest trauma suffer from rib fractures (35–40%) [4, 5], costal cartilage fractures (CCFX) are also frequently observed in high-energy trauma CT studies [6]. Fractured cartilage contributes to ribcage instability and may present as prolonged post-traumatic pain and discomfort even months after the initial trauma. Sonography may reveal a CCFX, but cross-sectional imaging (CT, MRI) is essential to verify the clinical suspicion of CCFX. Cartilage fractures are not visible on plain radiographs [7,8,9,10]. The healing process of CCFX is not well known; an animal study and a biomechanical study suggest that the union may be unstable [11, 12]. Some case reports revealed prolonged disability due to cartilage injury in contact-sport athletes [8, 13].
For this prospective study, the patients with diagnosed CCFX were summoned for clinical and radiological examinations to evaluate the timeline and natural course of healing. Post-traumatic clinical manifestations and radiological findings of 21 patients with CCFX were evaluated with multimodality imaging (ultra-low-dose CT, MRI, and US), clinical observation and inspection, and two quality of life (QoL) questionnaires. Trauma Registry data were explored to determine clinical variables for these patients. The main aim of this study was to evaluate long-term effects and manifestations of CCFX, to describe typical radiological findings, and to assess impact on QoL.
Background
Rib fractures are painful and may have an impact on respiratory function. During recuperation from chest trauma, the patients may experience difficulty in performing daily activities and loss of work ability [14]. Shallow breathing due to pain can lead to serious complications. To prevent pulmonary complications, such as respiratory failure, pneumonia, and empyema, aggressive pain management and respiratory support are recommended [15, 16]. These complications are more frequent in patients with multiple rib fractures, and particularly in those with flail chest [17]. The role of CCFX in ribcage instability, post-traumatic pain, and flail chest formation has remained unclear due to sparse data [6, 7, 9]. The general knowledge of CCFX has grown due to increased use of CT and MRI. Plain chest radiographs do not reveal CCFX, as cartilage tissue is radiolucent.
Many case reports in athletes have demonstrated detection of costal cartilage injuries with MRI [8, 10, 13, 18,19,20], but cartilage fractures are also clearly visible on CT (Fig. 1) [6, 9, 10]. In whole-body CTs for trauma (WBCT), cartilage injuries may remain overlooked, for example due to perceptual errors or satisfaction of search while reporting multiple injuries in polytrauma patients [6]. Although most blunt chest wall trauma is treated non-operatively, awareness of CCFX adds to the understanding of the extent and volume of thoracic injury and ribcage instability [10]. The late consequences of blunt chest trauma include pain, sense of ribcage stiffness, snapping sensation, loss of respiratory volume, and restrictions in physical activity [14]. At our institution, blunt chest trauma patients have clinical follow-up consultations with trauma surgeons 1 and 6 weeks after release from care. In general, follow-up imaging consists of plain radiographs; CT imaging is reserved for further evaluation of clinical complications.
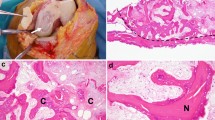
A 64-year-old male with a dislocated costal cartilage fracture on the left 1st rib (arrowhead) from a chest compression injury (left upper axial image). Also, a non-dislocated fracture on the right 1st-rib cartilage. Clinical examination revealed no tenderness on palpation around the fracture site, and the patient was asymptomatic. Ultra-low-dose CT (right upper axial image) reveals calcifications (arrowhead) on the fracture site and suggests a stable fracture union. T1-axial MR image (lower left image) shows the cartilage union (arrowhead). Note that there is no fatty infiltration in pectoral muscles. Ultrasound image of the same fracture site (lower right image) shows the step off
Materials and methods
Patients
This study was conducted in a level I trauma center in a metropolitan area of 1.8 million people. Institutional review board approval was granted by the Ethics Committee of the Department of Surgery for clinical follow-up, questionnaires, and imaging studies. In a previous retrospective study, the patients with CCFX were identified from WBCT examinations for trauma from the hospital picture archiving and communication systems, Agfa IMPAX 6.0 (Agfa HealthCare NV, Mortsel, Belgium). All the patients who entered the ER with a history of blunt trauma, were > 18 years of age, and underwent WBCT for trauma during the years 2013–2015 were included. From this study cohort, 114 patients with CCFX were identified. Of these, 8 patients died during the first 30 days after trauma [SI 1] and 5 died between the date of leaving the hospital and the beginning of data collection (15 April 2017). Additionally, 38 patients were not reachable due to location or health issues. The patients with pacemakers (2) were excluded due to restrictions in MRI imaging, as there was no cardiologist available on site to adjust the pacemaker during research imaging hours. In addition, 14 patients with CCFX identified from the year 2016 WBCTs for trauma were also invited to this prospective study to evaluate chronologically more recent fractures [SI 2].
Invitation letters were sent to 75 patients (61 [2013–2015] and 14 [2016]), 21 (28.0%) patients participated in this study (16 of 61, 26.2% and 5 of 14, 28.6%). The patients were invited via regular mail. The letter included a cover letter with a description of patient selection, basic principles of the study, and contact information; study information, and patient consent form approved by the ethics committee; a standardized RAND SF36 QoL questionnaire [21, 22]; a dedicated QoL questionnaire for patients with blunt thoracic trauma (designed by authors) [SI 3]; and an MRI safety questionnaire as an institutional standard procedure. All the patients had the right to discontinue the study and withdraw their consent; all enrolled subjects completed the entire study protocol.
Clinical control and Trauma Registry data
An orthopedic trauma surgeon with > 14 years of clinical experience evaluated the current physical status, disability, and ranges of movement (ROM). Ribcage symmetry and chest wall movements during the breathing cycle were visually examined. Breathing sounds were bilaterally evaluated. Entire chest wall, clavicles, scapulas, shoulder areas, and the injured parts of the chest wall were manually palpated to detect focal tenderness. The ROM in glenohumeral joints was analyzed and the underlying reason for possible diminished ROM was evaluated.
Trauma Registry data were explored to determine mechanism of injury (MOI), New Injury Severity Score (NISS), and Injury Severity Score (ISS), intensive care unit length of stay (ICU LOS), university hospital length of stay (LOS), need for mechanical ventilation, and duration of ventilation.
Imaging
The chest with focus on the area of injured cartilage (identified in the initial WBCTs for trauma) was imaged with all three different modalities for each patient as follows: (1) ultrasound (GE Logiq E9, GE Healthcare, Chicago, IL), (2) ultra-low-dose-CT; ULD-CT (Discovery 750HD, 64-detector row CT scanner, GE Healthcare), and (3) 1.5 T MRI (MAGNETOM Aera, 1.5 T, Siemens Healthcare GmbH, Erlangen, Germany). Imaging was performed without intravenous contrast media administration.
Ultrasound (US) images of the injured cartilage were obtained in conjunction with the physical examination. The following types of broad-spectrum linear transducers were used: 9L-D (2–8 MHz, FOV 44 mm) and 12L-RS (5–13 MHz, FOV 39 mm). US was used to identify the costochondral junction and the cartilage fracture site. Doppler US was used to identify increased vascularity at the fracture site. Sonar palpation was used to indicate movement in the fracture line.
A non-contrast ULD-CT (sub-mSv) protocol with iterative reconstruction (VEO, GE Healthcare) was used to ensure minimal radiation in imaging of a benign condition. Three-plane orthogonal reformatted image stacks (axial, sagittal, coronal) were used for evaluation.
MRI images (five sequences) were obtained in prone position (free respiration, non-gated) with a body-coil. Three sequences were imaged in the axial plane from the area of injured cartilage (T2-fat-saturated axial, T2 axial, and T1 axial). T1 sequence was used to evaluate fatty infiltration of the pectoralis muscles. Two sequences were obtained in the coronal plane of the anterior chest wall (STIR coronal or PD fat-saturated coronal and T2 coronal). The orientation was coronal oblique — parallel to the sternum (Appendix, Fig. 5). MRI imaging was performed by an experienced radiology technician and monitored directly by at least one of the radiologists involved in this study.
Image interpretation
US images were evaluated by the radiologist performing the examination. Both ULD-CT and MRI images were interpreted separately by two radiologists, who were blinded to each other and to physical exam results. Fracture healing was evaluated by assessment for noticeable fracture line, persistent edema on fracture site, presence and degree of calcification, and increased or decreased fracture dislocation. Equivocal findings were settled by consensus readout, which was used in the final analysis.
Quality of life questionnaires
Two survey instruments were used in this study to evaluate health-related QoL and degree of disability after trauma. All the patients participating in this study received a standardized RAND 36 questionnaire (36-Item Short Form Survey Instrument [SF-36]) [21, 22]. In addition, a dedicated thoracic trauma questionnaire [SI 3] was written to evaluate limitations and symptoms particularly due to thoracic injury. The results from the RAND 36 questionnaire were compared between the patients with healed and non-healed costal cartilage fractures.
Statistical analyses
The interobserver agreement for imaging findings was determined using Cohen’s kappa (κ). The κ-values were considered as follows: 0.01–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.80–0.99 almost perfect agreement. Proportion of agreement (POA) was also used to test agreement of dichotomous evaluation between readers (interobserver variation). The strength of POA was determined as follows: 0.0–0.2 poor, 0.21–0.4 fair, 0.41–0.6 moderate, 0.61–0.8 good, and 0.81–1.0 excellent [23].
Statistical analyses were performed using a commercial software package SAS/STAT v.9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
All the participants (21) were male, and age ranged from 30 to 77 (mean 59.8 years, median 63 years). The most common mechanism of injury was a fall from a height (7/21, 33.3%) (Tables 1 and 4). The mean time from injury to follow-up examination was 34.1 months (median 36, range 15.8–57.7). Fourteen (66.7%) had multiple costal cartilage fractures.
Clinical status
Four patients (19.0%) complained of persisting pain or discomfort at the fracture site. In 3 patients, fractures were evaluated as non-healed on CT, and these patients had focal tenderness on clinical exam. Ribcage asymmetry was found in 2 patients (9.5%). Seven patients (33.3%) reported focal tenderness on clinical examination. Fracture site was palpable in 7 patients (33.3%); 3 presented with focal tenderness. Two patients reported persistent pain from scapular fracture and 1 from multiple rib fractures. Two patients had a diminished ROM, 1 in the entire rib cage, and the other due to scapular fracture. There were no clinical signs of restricted respiration and no signs of involvement of accessory breathing muscles (Table 2).
Imaging
All the patients underwent all three different modalities in follow-up imaging. The imaging intervals are stated in Table 4. Most costal cartilage fractures (15/21, 71.4%) were radiologically evaluated as healed (κ 0.69 (95% CI 0.3771–1.000) (Fig. 3). The results from the image evaluation are shown in Table 3.
Sonography was most useful in dynamic evaluation and detected cartilage movement and dislocated fractures (Appendix, Fig. 3). Instability was perceived on dynamic US and during clinical examination in 1 patient (4.7%). Movement was detected in dynamic US examination while compressing the fracture area with the US probe. First-rib costochondral synostosis was not an optimal site for US examination due to its location deep in the clavicula and sternoclavicular joint and since the first rib cartilages are usually heavily calcified on the surface. No extra benefit from the use of Doppler US was noted, and no vascular changes in the damaged areas were detected.
Initially, fractures were detected in 10 patients (47.6%). Ultra-low-dose-CT examination showed calcifications around the initial fracture site in all the patients (Fig. 1). These post-traumatic calcifications differ from physiological cartilage calcifications in morphology and orientation (Appendix, Fig. 4). Post-traumatic calcifications are found vertically oriented in the fracture line and horizontally following the perichondrium. The physiological calcifications are patchy, more randomly distributed, and parallel to the long axis of the rib.
In comparison with the initial trauma CT study, unsuspected increased dislocation of the evaluated or adjacent CCFX was found in 5 patients (23.8%). Initially, dislocated fractures were found in 10 patients (47.6%) (Table 4). ULD CT imaging of the thorax had a mean dose length product (DLP) of 32 (range 15–58) when compared to the initial trauma WBCT DLP of 1668 (range 516–4486) (Table 4). Ultra-low-dose imaging provided sufficient image quality for evaluating the post-traumatic changes of the bony ribcage (Fig. 1). Fracture lines remained visible on CT in 6 patients (28.6%).
MRI image acquisition was performed with a body-coil during free breathing. The patients were lying face down to reduce anterior chest wall movement artifacts. Only one patient reported discomfort at the end of the series of images taken in prone position. All the adequate images had been taken at that point. T2-weighted images were considered most useful in evaluating cartilage and their continuity (Fig. 2). Fat-saturated T2-weighted images showed persistent edema at the fracture site in 8 patients (38.1%) (Fig. 2). No atrophy or fatty degeneration of the pectoralis muscles was detected on T1-weighted images (Fig. 1).
Quality of life questionnaires
No statistically significant difference of RAND36 scores between radiologically healed and non-healed patients was detected. In the dedicated chest trauma survey, 9 patients (42.9%) reported no thoracic pain. In 7 patients (33.3%), thoracic pain affected sleep, while in 13 (61.9%) pain from other body regions affected sleep.
Most patients had chest-related symptoms. The most common symptoms were chronic cough (14 patients, 66.7%), difficulties with deep inhalation (12 patients, 57.1%), difficulties with strenuous exercise (12 patients, 57.1%), local pain (7 patients, 33.3%), tingling or numbness (5 patients, 23.8%), and a snapping sensation (5 patients, 23.8%). Ten (47.6%) patients used pain medication daily, most commonly paracetamol (5 patients, 23.8%) and NSAIDs (4 patients, 19.0%). Four (19.0%) patients reported no pain at all. Other symptoms affecting daily life were pain or dysfunction of the lower extremities in 7 patients (33.3%) and spine in 9 patients (42.9%).
Trauma Registry data
Seven patients (33.3%) were not included in the Trauma Registry as their ISS score ≤ 16. For the remaining 14 patients (66.7%), mean NISS score was 31.9 (median 34, range 17–43) and mean ISS score was 25.5 (median 25.5, range 17–38). The mean ICU length of stay (ICU-LOS) was 8.6 days (median 8.5, range 1–17). Mean university hospital LOS was 14.6 days (median 13, range 7–30). Eight of these 14 patients (57.1%) needed mechanical ventilation for mean duration of 8.5 days (range 1–16) (Table 1).
Discussion
Detection of costal cartilage fractures has increased due to increasing use of cross-sectional imaging by CT and MRI. As a result of the healing process, calcifications commonly develop at the fracture site and are visible on CT. Fracture dislocation can increase over time and persistent movement can be detected on dynamic US. MRI is best suited for visualizing local persistent edema. Clinical examination reveals focal tenderness and a palpable fracture site. In general, most CCFX will heal over time and become asymptomatic. However, some patients report pain, discomfort, and a snapping sensation around the costal cartilage fracture even years after the initial trauma. A diagnostic work-up that consists of both clinical examination and imaging with CT or MRI ensures a comprehensive evaluation. Most, but not all, fractures that were radiologically evaluated as non-healed were symptomatic. Conversely, some symptomatic fractures were evaluated as healed on imaging. Thus, the combination of clinical examination and imaging will help assess the overall situation.
As costal cartilage injuries are commonly seen in high-energy trauma, other concomitant injuries add to the pain and disability load, and cartilage injuries may remain overlooked as the source of pain and discomfort.
This study had some limitations. Traumatic changes were not limited to the thorax; most patients had additional severe injuries. Only patients without significant physical disability accepted the invitation and were able to attend the appointments without any assistance. There is a possibility that patients who were more severely affected or restricted by their injuries might have chosen to not participate in this follow-up study, implicating selection bias. Another bias was created from the motivation of the patients to participate. Most of the patients who decided to participate in this study had prolonged symptoms from the original trauma; some had symptoms outside the thoracic region. As the main aim of this study was to examine the healing process of CC fractures and describe the clinical and radiological post-traumatic changes, any patient that was previously diagnosed with a costal cartilage injury was considered valuable to this study. The number of potential participants for the study was limited mostly by injury incidence and patient’s willingness to participate.
Follow-up imaging was performed on average 3 years after the initial trauma, which we consider sufficient for the evaluation of long-term effects. This interval was an estimate based on the general experience of trauma radiologists and trauma surgeons included in this study with reference to how osseous rib fractures tend to heal. A more dedicated follow-up protocol with preset intervals for follow-up imaging (e.g., 3, 6, 12, and 24 months) may provide more detailed information about the healing process. We recommend that in patients with persistent symptoms (pain, discomfort) in the anterior chest wall, MRI, and CT follow-up imaging would give information about the current status of the fractures. Clinical examination made at the same time would provide additional information. In selected cases with severely dislocated/unstable fractures, operative treatment might be considered.
For this study, we chose ULD-CT, MRI, and US for imaging because of their respective strengths and to compensate for weaknesses. Different imaging modalities provide complementary advantages regarding cartilage injury diagnosis and follow-up. ULD-CT has a very low radiation dose but is well suited for evaluating degree of dislocation and presence of cartilage calcifications. Overall, CT visualizes osseous and cartilaginous structures and fracture lines very well, whereas fracture instability can be visualized on dynamic US. MRI is best suited for evaluating soft-tissue changes such as edema and muscle atrophy. Movement artifacts may affect image quality on MRI, which is dependent largely on patient co-operation, scanners, and imaging protocols.
POA in addition to κ were used to express interobserver agreement, as κ is affected by the prevalence of the finding under observation. For rare findings, such as in “no fracture line visible on MRI T2” in this study, very low κ values may not necessarily reflect low rates of overall agreement [24, 25]. The interobserver agreement and proportion of agreement were good on ULD-CT studies.
Visible fracture line can be detected on US, CT, or MRI. MRI shows persistent edema on the fracture site and possible dislocation. T1-weighted images illustrate fatty degeneration or atrophy of muscles. The primary value of US was in detecting fracture instability on dynamic examination. US is inherently observer-dependent, which necessarily affects reproducibility. Standardized imaging planes on CT and MRI avoid this limitation by providing easily comparable imaging data.
Conclusions
Non-union in CCFX is uncommon but may lead to decreased stability and discomfort. Both clinical and radiological examinations play an important part in the post-traumatic evaluation of CCFX. CT and MRI visualize the healing process, while dynamic ultrasound may reveal instability. No significant difference in QoL was detected between the patients with radiologically healed and non-healed CCFX. Post-traumatic disability was mostly due to other non-thoracic injuries.
References
Bulger EM, Arneson MA, Mock CN, Jurkovich GJ (2000) Rib fractures in the elderly. J Trauma 48(6):1040–1046. https://doi.org/10.1097/00005373-200006000-00007
Dennis BM, Bellister SA, Guillamondegui OD (2017) Thoracic trauma. Surg Clin North Am 97(5):1047–1064. https://doi.org/10.1016/j.suc.2017.06.009
Holcomb JB, McMullin NR, Kozar RA, Lygas MH, Moore FA (2003) Morbidity from rib fractures increases after age 45. J Am Coll Surg 196(4):549–555
Pope TL (2013) Harris & Harris’ The radiology of emergency medicine, 5th edn. Lippincott Williams & Wilkins, Philadelphia [cited 2022 Jun 3]. Available from: https://search.ebscohost.com/login.aspx?direct=true&db=e000xww&AN=1473176&site=ehost-live&scope=site
Mirvis SE (2004) Diagnostic imaging of acute thoracic injury. Semin Ultrasound CT MR 25(2):156–179
Nummela MT, Bensch FV, Pyhältö TT, Koskinen SK (2018) Incidence and imaging findings of costal cartilage fractures in patients with blunt chest trauma: a retrospective review of 1461 consecutive whole-body ct examinations for trauma. Radiology 286(2):696–704
Subhas N, Kline MJ, Moskal MJ, White LM, Recht MP (2008) MRI Evaluation of costal cartilage injuries. Am J Roentgenol Am Roentgen Ray Soc 191:129–132
Lopez V, Ma R, Li X, Steele J, Allen AA (2013) Costal cartilage fractures and disruptions in a rugby football player. Clin J Sport Med 23(3):232–234
Malghem J, Vande Berg B, Lecouvet F, Maldague B (2001) Costal cartilage fractures as revealed on CT and sonography. AJR Am J Roentgenol 176(2):429–432
McAdams TR, Deimel JF, Ferguson J, Beamer BS, Beaulieu CF (2016) Chondral rib fractures in professional American football: two cases and current practice patterns among NFL team physicians. Orthop J Sport Med 4(2):2325967115627623
Piao Z, Takahara M, Harada M, Orui H, Otsuji M, Takagi M et al (2007) The response of costal cartilage to mechanical injury in mice. Plast Reconstr Surg 119(3):830–836
Forman JL, Kent RW (2014) The effect of calcification on the structural mechanics of the costal cartilage. Comput Methods Biomech Biomed Engin 17(2):94–107
Hayashi D, Roemer FW, Kohler R, Guermazi A, Gebers C, De Villiers R (2014) Thoracic injuries in professional rugby players: mechanisms of injury and imaging characteristics. Br J Sports Med 48(14):1097–1101
Fabricant L, Ham B, Mullins R, Mayberry J (2013) Prolonged pain and disability are common after rib fractures. Am J Surg 205(5):511–516
Pressley CM, Fry WR, Philp AS, Berry SD, Smith RS (2012) Predicting outcome of patients with chest wall injury. Am J Surg 204(6):910–914
Flagel BT, Luchette FA, Reed RL, Esposito TJ, Davis KA, Santaniello JM et al (2005) Half-a-dozen ribs: the breakpoint for mortality. Surgery 138(4):717–725
Ciraulo DL, Elliott D, Mitchell KA, Rodriguez A (1994) Flail chest as a marker for significant injuries. J Am Coll Surg 178(5):466–470
Peterson LL, Cavanaugh DG (2003) Two years of debilitating pain in a football spearing victim: slipping rib syndrome. Med Sci Sports Exerc 35(10):1634–1637
Willis-Owen C, Kemp SP, Thomas RD (2009) Hepatic injury after costochondral separation in a rugby football player. Clin J Sport Med 19(1):70–71
Kemp SP, Targett SG (1999) Injury to the first rib synchondrosis in a rugby footballer. Br J Sports Med 33(2):131–132
Laucis NC, Hays RD, Bhattacharyya T (2014) Scoring the SF-36 in orthopaedics: a brief guide. J Bone Jt Surg - Am 97(19):1628–1634
Patel AA, Donegan D, Albert T (2007) The 36-item short form. J Am Acad Orthop Surg 5(2):126–134. https://doi.org/10.5435/00124635-200702000-00007
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Feinstein AR, Cicchetti DV (1990) High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol 43(6):543–9
Cicchetti DV, Feinstein AR (1990) High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol 43(6):551–558
Acknowledgements
Our deepest gratitude goes to radiology technician Jaana Koskiniemi for her dedication and for performing all prospective MRI studies and to all radiology technicians and department secretaries in Töölö Trauma Center for their skillfull assistance in this study. We also thank trauma registry nurses Satu Tirkkonen, Markku Kytönen, Iiu Laitinen, Marja Bergman, and Pirkko Tonder for accurately collecting the Helsinki Trauma Registry data.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare regarding this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nummela, M.T., Pyhältö, T.T., Bensch, F.V. et al. Costal cartilage fractures in blunt polytrauma patients — a prospective clinical and radiological follow-up study. Emerg Radiol 29, 845–854 (2022). https://doi.org/10.1007/s10140-022-02066-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-022-02066-w