Abstract
Tetrodotoxin (TTX), known as pufferfish toxin, is a potent neurotoxin blocking sodium channels in muscle and nerve tissues. TTX has been detected in various taxa other than pufferfish, including marine polyclad flatworms, suggesting that pufferfish toxin accumulates in fish bodies via food webs. The composition of TTX and its analogs in the flatworm Planocera multitentaculata was identical to those in wild grass puffer Takifugu alboplumbeus. Previously, Planocera sp. from Okinawa Island, Japan, were reported to possess high level of TTX, but no information was available on TTX analogs in this species. Here we identified TTX and analogs in the planocerid flatworm using high-resolution liquid chromatography–mass spectrometry, and compared the composition of TTX and analogs with those of another toxic and non-toxic planocerid species. We show that the composition of TTX and several analogs, such as 5,6,11-trideoxyTTX, dideoxyTTXs, deoxyTTXs, and 11-norTTX-6(S)-ol, of Planocera sp. was identical to those of toxic species, but not to its non-toxic counterpart. The difference in the toxin composition was reflected in the phylogenetic relationship based on the mitochondrial genome sequence. A toxification experiment using predatory fish and egg plates of P. multitentaculata demonstrated that the composition of TTX and analogs in wild T. alboplumbeus juveniles was reproduced in artificially toxified pufferfish. Additionally, feeding on the flatworm egg plates enhanced the signal intensities of all TTX compounds in Chelonodon patoca and that of deoxyTTXs in Yongeichthys criniger.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tetrodotoxin (TTX), known as pufferfish toxin, is one of the most potent natural toxins, inhibiting neurotransmission by blocking sodium channels in muscle and nerve tissues (Narahashi 2001). TTX is found in a wide variety of marine organisms that are prey of pufferfish, such as gastropods, crustaceans, ribbonworms, and flatworms (Noguchi et al. 2006; Noguchi and Arakawa 2008; Bane et al. 2014; Magarlamov et al. 2017; Katikou et al. 2022). In addition, non-toxic pufferfish can be produced by rearing them on TTX-free feed, and these non-toxic pufferfish rapidly toxify when fed TTX-containing feed, suggesting that TTX in wild pufferfish tissues is derived from prey organisms (Matsui et al. 1981, 1982; Honda et al. 2005; Noguchi et al. 2006; Itoi et al. 2015). However, although the biosynthetic mechanism of TTX has been inferred from the discovery of various TTX analogs (Kudo et al. 2014a, b, 2016, 2020, 2021; Ueyama et al. 2018; Kudo and Yotsu-Yamashita 2019), there is no information on the gene cluster involved in this process.
The search for missing links among TTX-bearing organisms, especially relationships relative to pufferfish, leads to TTX-bearing flatworms. For example, it has been shown that the toxification of the sea slug Pleurobranchaea maculata, the causative organism of dog poisoning in New Zealand, resulted from feeding on the flatworm Stylochoplana sp. (Salvitti et al. 2015). It has been also shown that grass puffer Takifugu alboplumbeus ingested TTX by feeding on the flatworm Planocera multitentaculata throughout its life history (Itoi et al. 2018; Okabe et al. 2019), and that juveniles of milk-spotted puffer Chelonodon patoca and toxic goby Yongeichthys criniger ingested TTX by feeding on larvae of P. multitentaculata or related species (Itoi et al. 2020a; Ito et al. 2022). Furthermore, planocerid flatworm larvae have been reported to be involved in the toxification of bivalves with TTX (Okabe et al. 2021), which has become a worldwide problem in recent years (EFSA Contam Panel et al. 2017; Biessy et al. 2019; Antonelli et al. 2022; Yasukawa et al. 2023).
No TTX-free individuals were detected in the flatworm P. multitentaculata, and that the amount of TTX in the body increased with body size (Yamada et al. 2017; Itoi et al. 2020b). This flatworm is recognized as an important key organism in the investigation of TTX origin. Recently, it has been reported that P. multitentaculata possess a variety of TTX analogs, including dideoxyTTXs, deoxyTTXs, and 11-norTTX-(S)-ol, in addition to large amounts of TTX and 5,6,11-trideoxyTTX (Suo et al. 2022). Although the internal localization of these TTX analogs in the flatworm changes during growth (Oyama et al. 2022), there is no difference in the composition of TTX analogs between samples collected in different sites (Suo et al. 2024), indicating that the flatworm might possess biosynthetic processes of TTX in the body. On the other hand, there is no information on TTX analogs in planocerid flatworms, with the exception of the planocerid in Guam (Ritson-Williams et al. 2006) and P. multitentaculata as mentioned (Suo et al. 2022, 2024).
The 5,6,11-trideoxyTTX has been reported to be dominantly high in a variety of organisms (Rambla-Alegre et al. 2017; Suo et al. 2021, 2022, 2024; Ito et al. 2022, 2023; Vlasenko and Magarlamov 2020, 2022). Recently, TTX/5,6,11-trideoxyTTX ratios of pufferfish, including C. patoca, collected throughout the Japanese Archipelago, ranged from 2 to 5 regardless of species or region. Meanwhile, the ratios within toxic goby Y. criniger collected in Okinawa and Ishigaki Island, including individuals collected at the same sites as C. patoca, were found to differ markedly depending on the date/location of sampling (Ito et al. 2022, 2023). This suggests not only that C. patoca and Y. criniger feed on different organisms but also that the accumulation mechanism of TTX analogs differs between them and that the composition of TTX analogs in the bodies of Y. criniger is affected by the composition of TTX analogs in their prey.
In this study, we sought to accumulate the basic knowledge required to elucidate the source of TTX and its analogs in pufferfish and toxic goby. Additionally, we attempted to reveal the composition of TTX and its analogs in four species of Japanese planocerid, and to clarify the factors affecting changes in the composition of TTX and analogs in pufferfish and toxic goby after feeding on the egg plates of P. multitentaculata.
Materials and Methods
Flatworms
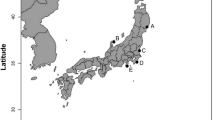
Four species of planocerid flatworms were collected from various locations in the Japanese Islands. Planocera sp. (Fig. 2a) were collected in August, 2017, under stones at a depth of 10 m off Nago, Okinawa Island in the Ryukyu Islands, Japan (“B” in Fig. 1; Ueda et al. 2018). Planocera reticulata (Fig. 2b) and Planocera pellucida (Fig. 2d) were collected in May, 2023, and May, 2019, respectively, under stones in coastal waters around Miura Peninsula, Kanagawa, Japan (“E” in Fig. 1), and Planocera multitentaculata (Fig. 2c) were collected in June, 2020, at Ibaraki, Japan (“F” in Fig. 1). These samples were stored at – 30 °C until use. Several specimens of P. multitentaculata were also collected in April, 2022, around Miura Peninsula, Kanagawa, Japan, and reared in a 60-L tank to collect egg plates for use in toxification experiment.
Fish Juveniles
TTX-bearing juvenile fish (analyzed as wild individuals) were collected from various locations in the Ryukyu Islands, Japan as follows: juveniles of Chelonodon patoca (mean body weight 1.02 ± 0.44 g, n = 10) and Yongeichthys criniger (mean body weight 0.65 ± 0.26 g, n = 13) were collected on Ishigaki Island (“A” in Fig. 1) in June, 2022, whereas Takifugu alboplumbeus (mean body weight 0.84 ± 0.23 g, n = 10) were collected from Okinawa Island (“C” in Fig. 1) in May, 2022. These samples were stored at – 30 °C until analysis.
Juvenile fish used in the toxification experiments were prepared as follows: non-toxic juveniles of T. alboplumbeus (mean body weight 0.16 ± 0.06 g, n = 11) were raised using commercial feed pellets after artificial fertilization at Enoshima, Kanagawa, Japan (“D” in Fig. 1) in June, 2022, whereas juveniles of C. patoca (mean body weight 0.47 ± 0.13 g, n = 9) and Y. criniger (mean body weight 0.37 ± 0.18 g, n = 10) were collected on Ishigaki Island (“A” in Fig. 1) in June, 2022, and reared in laboratory tanks on non-toxic feed for 1 month. Non-toxic juveniles of T. alboplumbeus used in the intramuscular administration of TTX (mean body weight 1.16 ± 0.06 g, n = 3) or 5,6,11-trideoxyTTX (mean body weight 1.59 ± 0.15 g, n = 3) were artificially raised in 2023 in a similar procedure to that used for the toxification experiments.
Toxification Experiment
To reproduce the composition of TTX and analogs in predators feeding on TTX-bearing flatworms, we conducted toxification experiments in which T. alboplumbeus, C. patoca, and Y. criniger were fed egg plates of P. multitentaculata, under the following conditions. Juvenile fish were housed individually in mesh-partitioned cages (70 mm length × 110 mm width × 70 mm depth; up to 12 compartments) within 15-L tanks under aeration. Fish were fed a flatworm egg plate (approx. 0.08 g) containing approx. 190 μg TTX and 180 μg 5,6,11-trideoxyTTX for 1 day (Ito et al. 2023). After the treatment, individuals of all three fish species were reared for 1 week on non-toxic commercial feed pellets, allowing for excretion of toxic flatworm egg plates. The composition of TTX and analogs in these juvenile fish was then measured by liquid chromatography–mass spectrometry (LC–MS) analysis (Suo et al. 2022).
Intramuscular Administration
TTX and 5,6,11-trideoxyTTX were dissolved in 0.1% acetic acid, and fish were intramuscularly injected with TTX or 5,6,11-trideoxyTTX at a dose of 6 μg/individuals. TTX for use in calibration solution for quantification of TTX was purchased from FUJIFILM Wako Pure Chemicals (purity ≥ 90%), while 5,6,11-trideoxyTTX was purified in accordance with Miyazaki et al. (2024). The fish injected with 0.1 mL of acetic acid were used as control group. After the treatment, all individuals were reared for 1 week on non-toxic commercial feed pellets. The composition of TTX and analogs in these fish was then measured LC–MS analysis (Suo et al. 2022).
Extraction of TTX and Its Analogs
Extracts from planocerid flatworms and juvenile fish containing TTX and its analogs were prepared in accordance with Suo et al. (2022). Flatworm tissue was homogenized in 0.01 M acetic acid followed by heating for 10 min in boiling water. After centrifugation at 12,000 × g for 5 min at 4 °C, the supernatant was recovered and filtered through a membrane of pore size 0.45 μm. The filtrate was adjusted to pH 6.0 with acetic acid and loaded onto an activated charcoal column equilibrated with water. After washing the column with water, the TTX and analogs were eluted with acetic acid/ethanol/water (2:50:49, v/v), and the recovered solution was concentrated and lyophilized. The residue was redissolved in an acid solution adjusted to pH 6.0, and loaded onto the Bio-Gel P-2 column equilibrated with water. After washing the column with water, the compounds were eluted with 0.03 M acetic acid, and the fractions containing TTX and analogs were combined and concentrated for LC–MS analysis.
LC–MS Analysis
The prepared extracts from flatworms and juvenile fish were appropriately diluted and subjected to high-resolution LC–MS analysis according to the method of Suo et al. (2022). LC–MS was carried out on a Shimadzu LC-20AD solvent delivery system connecting to a SCIEX X500R Q-TOF mass spectrometer with an ESI source. The interface parameters for optimized conditions were set as follows: curtain gas, 45 psi; ion spray voltage, 5500 V; temperature, 700 °C; ion source gas 1, 70 psi; ion source gas 2, 60 psi; collision gas, 7 psi. The TTX standards and the flatworm sample extracts were analyzed by LC–MS using a TSKgel Amide-80 column (2.0 mm × 150 mm, 5 μm) with 16 mM ammonium formate in water/acetonitrile/formic acid (30:70:0.002, v/v) at a flow rate of 0.2 mL/min at 28 °C (Jang et al. 2010). The [M + H]+ ions were measured using the extracted-ion chromatography XIC (± 0.02) corresponding ions for 5,6,11-trideoxyTTX at m/z 272.1241, dideoxyTTXs at m/z 288.1190, 11-norTTX-6(S)-ol at m/z 290.0983, deoxyTTXs at m/z 304.1139, and TTX and 4-epiTTX at m/z 320.1088. TTX for use in calibration solution for quantification of TTX was purchased from FUJIFILM Wako Pure Chemicals (purity ≥ 90%). Chemically synthesized 5,6,11-trideoxyTTX (Adachi et al. 2014) was provided as an authentic preparation. The standard calibration curve was created using 1–100 ng/mL of standards (TTX and 5,6,11-trideoxyTTX) which showed good linearity and precision (TTX: y = 9952.5x, R2 = 0.99; 5,6,11-trideoxyTTX: y = 5898.2x, R2 = 0.97). The limit of detection (LOD) was determined based on signal to noise ratio (S/N: 3). LODs of TTX and 5,6,11-trideoxyTTX were 0.2 and 0.8 ng/mL, respectively.
DNA Extraction, Sequencing, and Phylogenetic Analysis
Total genomic DNA was extracted from tissue using QIAamp FFPE Tissue Kit (Qiagen, Hilden, Germany) for two individuals of Planocera sp., DNeasy Blood & Tissue Kit (Qiagen) for three individuals of P. pellucida, and NucleoBond HMW DNA (Macherey–Nagel, Düren, Germany) for one individual of P. pellucida. Libraries were prepared using MGIEasy FS DNA Library Prep Set (MGI, Shenzhen, China) for two individuals of Planocera sp. and one individual of P. pellucida, and Nextera DNA Flex library Prep kit (Illumina, San Diego, CA, USA) for three individuals of P. pellucida. Next-generation sequencing analysis was performed using DNBSEQ-T7 (MGI) for two individuals of Planocera sp., HiSeqX (Illumina) and DNBSEQ-G400 (MGI) for three and one individual(s) of P. pellucida, respectively. Full sequences of mitochondrial DNA were constructed by mapping short reads to that of P. multitentaculata (LC503532) as a reference using CLC Genomics Workbench ver. 8.0.1 (Qiagen). Gene annotation including rRNA estimation and tRNA prediction was carried out using Geneious Prime Java ver. 11.0.14.1 + 1 (Biomatters Ltd., Auckland, New Zealand) and MITOS2 (Donath et al. 2019) according to Yonezawa et al. (2020). The complete mitochondrial DNA sequences in this study were submitted to the DDBJ/EMBL/GenBank databases with accession numbers LC785386–LC785391, respectively.
Comparison of gene arrangement in the complete mitochondrial DNA sequence was manually performed using schematic diagrams based on annotated sequences. The phylogenetic tree was constructed by means of the maximum likelihood method using MEGA X (Kumar et al. 2018). Nucleotide sequences of complete mitochondrial DNA for the following species were also obtained from the databases: Prosthiostomum siphunculus (KT363736), Enchiridium sp. (KT363734), Miroplana shenzhensis (NC_062124), Dugesia japonica (AB618487), Girardia sp. (KP090061), Diversibipalium sp. (MZ561470), and Bipalium adventitium (MZ561467). Echinococcus canadensis (AB208063) was used as the outgroup species. Phylogenetic trees were also constructed using partial mitochondrial DNA sequences including cytochrome b and cytochrome c oxidase subunit I (COI) genes.
Results
Phylogenetic Relationship of Planocerid Flatworms
Complete mitochondrial DNA sequences obtained from Planocera sp. and P. pellucida demonstrated that the gene arrangement was different between toxic and non-toxic planocerids (Fig. S1). The tRNA-Gln gene was arranged between NADH dehydrogenase subunit 5 (ND5) and tRNA-Ser genes in TTX-bearing planocerid species including P. multitentaculata and P. reticulata, whereas it was detected in the position between tRNA-Leu and tRNA-Lys genes in P. pellucida, which correspond to positions seen in other non-toxic flatworms.
Phylogenetic analysis was performed using these complete mitochondrial DNA sequences, along with previously reported flatworm sequences. The maximum likelihood tree showed that Planocera sp. and P. pellucida clustered with other planocerid flatworm species, P. multitentaculata and P. reticulata, with high bootstrap support (Fig. 3). However, the TTX-bearing flatworm Planocera sp. clustered with P. multitentaculata and P. reticulata, diverging at the root with the non-toxic P. pellucida. The phylogenetic tree based on sequences of cytochrome b and COI genes also showed that within Acotylea, non-toxic and toxic planocerid species diverged and Planocera sp. formed a cluster with toxic species (Fig. S2).
Maximum likelihood tree of the planocerid flatworms and related species in Platyhelminthes inferred from an alignment of complete mitochondrial DNA sequences. Numbers at branches denote the bootstrap percentages from 1000 replicates. The accession numbers LC785386–LC785388 shown in parentheses have been deposited in the DDBJ/EMBL/GenBank databases. Echinococcus canadensis was used as the outgroup. Only bootstrap values exceeding 50% are presented. The scale refers to nucleotide substitutions per site
Composition of TTX and Analogs from Four Planocerid Flatworms
As shown in Fig. 4, peaks for TTX and several compounds related to TTX were commonly detected by LC–MS analysis in two individuals of Planocera sp. corresponding to those seen in P. multitentaculata. The peak at 15.2 min in m/z 320.1088 was identical to TTX with 4-epiTTX, one of the chemically equilibrium molecules of TTX, at 13.5 min. The peak at 5.5 min in m/z 272.1241 was identical to 5,6,11-trideoxyTTX, while that at 12.4 min in m/z 290.0983 was identical to 11-norTTX-6(S)-ol. In addition, peaks at 10.3 and 13.1 min in m/z 304.1139 were identical to 11-deoxyTTX and 6-deoxyTTX, respectively. Peaks at 6–9 min in m/z 288.1190 would be considered the ions identical to 5,11-dideoxyTTX and 6,11-dideoxyTTX. Similar patterns for TTX and related compounds were also observed in P. reticulata. On the other hand, no peaks for TTX and related compounds were detected in P. pellucida.
Comparison of extracted ion chromatograms for tetrodotoxin and its analogs from Planocera sp. (S1 and S2), Planocera reticulata, Planocera multitentaculata, and Planocera pellucida. Panels for m/z 320.1088: TTX and 4-epiTTX; m/z 272.1241: 5,6,11-trideoxyTTX; m/z 290.0983: 11-norTTX-6(S)-ol; m/z 304.1139: deoxyTTXs; m/z 288.1190: dideoxyTTXs. cps, counts per second
Effects of Toxification with Planocera multitentaculata Egg Plates on Composition of TTX and Analogs in Predatory Fish
Composition of TTX and analogs in Planocera sp. was identical to that of P. multitentaculata, as described above. In addition, the composition of TTX and analogs in adult P. multitentaculata individuals was qualitatively similar to those of P. multitentaculata egg plates (Fig. S3), as reported in our previous study (Suo et al. 2022). Therefore, toxification experiments were conducted using egg plates of P. multitentaculata as the prey for predatory pufferfish and goby. The toxification experiment using the cultured pufferfish T. alboplumbeus juveniles demonstrated that the composition of TTX and analogs in artificially toxified juveniles was nearly identical to that of wild juveniles collected at Okinawa Island (Fig. 5). In addition, feeding on P. multitentaculata egg plates after rearing with a non-toxic diet for 1 month enhanced the signal intensities of all TTX-related substances in C. patoca juveniles, but only deoxyTTXs in Y. criniger juveniles (Fig. 6).
Effects of toxification using the toxic flatworm Planocera multitentaculata egg plate on the composition of tetrodotoxin (TTX) in the pufferfish Takifugu alboplumbeus. Extracted ion chromatograms represent TTX and its analogs from wild, toxified, and cultured T. alboplumbeus individuals. Wild pufferfish juveniles were collected from Okinawa Island, Japan (C in Fig. 1), whereas non-toxic fish juveniles were raised with commercial feed pellets after artificial fertilization at Enoshima, Japan (D in Fig. 1). Culture pufferfish juveniles were toxified by feeding on the flatworm P. multitentaculata egg plates. Panels for m/z 320.1088: TTX and 4-epiTTX; m/z 272.1241: 5,6,11-trideoxyTTX; m/z 290.0983: 11-norTTX-6(S)-ol; m/z 304.1139: deoxyTTXs; m/z 288.1190: dideoxyTTXs. cps, counts per second
Changes in the composition of tetrodotoxin (TTX) and its analogs in TTX-bearing fish juveniles in association with feeding on the toxic flatworm Planocera multitentaculata egg plates. Extracted ion chromatograms represent TTX and analogs from the pufferfish Chelonodon patoca (after/before toxification), toxic goby Yongeichthys criniger (after/before toxification), and egg plates of the flatworm Planocera multitentaculata. Juvenile fish collected at Okinawa Island (C in Fig. 1) were used for toxification experiments after rearing for 1 month with non-toxic commercial pellets. Panels for m/z 320.1088: TTX and 4-epiTTX; m/z 272.1241: 5,6,11-trideoxyTTX; m/z 290.0983: 11-norTTX-6(S)-ol; m/z 304.1139: deoxyTTXs; m/z 288.1190: dideoxyTTXs. cps, counts per second
A single component of TTX and 5,6,11-trideoxyTTX was detected in cultured T. alboplumbeus juveniles intramuscularly injected with TTX and 5,6,11-trideoxyTTX, respectively (Fig. 7).
Extracted ion chromatograms for TTX and its analogs in extractions from the pufferfish Takifugu alboplumbeus juveniles injected muscularly with TTX and 5,6,11-trideoxyTTX. Extracted ion chromatograms represent TTX and analogs from the TTX-injected pufferfish (skin), pure TTX used injection, 5,6,11-trideoxyTTX-injected pufferfish, and pure 5,6,11-trideoxyTTX used injection (skin). Panels for m/z 320.1088: TTX and 4-epiTTX; m/z 272.1241: 5,6,11-trideoxyTTX; m/z 290.0983: 11-norTTX-6(S)-ol; m/z 304.1139: deoxyTTXs; m/z 288.1190: dideoxyTTXs. cps, counts per second
Discussion
Extensive amounts of TTX have been thought to accumulate in the body of pufferfish via the food chain with bacteria as primary producers (Noguchi et al. 2006; Noguchi and Arakawa 2008), but many missing links among organisms leading to pufferfish have been unexplored. Recently, it has been shown that T. alboplumbeus efficiently ingests TTX from P. multitentaculata throughout their life history (Itoi et al. 2018; Okabe et al. 2019). It is known that P. multitentaculata possesses large amounts of TTX (Miyazawa et al. 1986; Yamada et al. 2017; Itoi et al. 2020b), and with the amount of TTX in the body dependent on body size (Yamada et al. 2017; Itoi et al. 2020b). A similar trend was observed in P. reticulata, although data are limited (Itoi et al. 2020b). Planocerid and closely related flatworms, mainly P. multitentaculata, have been reported to supply TTX to TTX-bearing marine organisms: Stylochoplana sp. to sea slug P. maculata (Salvitti et al. 2015); P. multitentaculata or closely related flatworms to C. patoca and Y. criniger (Itoi et al. 2020a); and P. multitentaculata to the akazara scallop Azumapecten farreri akazara (Okabe et al. 2021) and pufferfish of the genus Takifugu (Itoi et al. 2018; Okabe et al. 2019; Ito et al. 2023). However, these manifested relationships would be just the tip of the iceberg, as there are few examples of studies focusing on flatworms.
In the present study, it was shown that compositions of TTX and analogs in Planocera sp. and P. reticulata were identical to those seen in P. multitentaculata (Suo et al. 2022, 2024). On the other hand, none of the TTX and analogs were detected in P. pellucida, which is closely related to these toxic flatworms but is not known to possess TTX (Kashitani et al. 2020). Comparison of mitochondrial gene arrangement demonstrated a difference between toxic and non-toxic groups. Phylogenetic analysis based on mitochondrial DNA sequences showed that these TTX-bearing flatworms in the genus Planocera form a cluster diverged from non-toxic planocerids at the root, suggesting that these toxic planocerids may possess the gene clusters necessary for the possession/accumulation of TTX (Kashitani et al. 2020), and these toxic species may be potential TTX sources in marine environments. Furthermore, since TTX was also detected in several flatworm species, including marine and terrestrial flatworms (Tanu et al. 2004; Stokes et al. 2014; Salvitti et al. 2015; Kashitani et al. 2020; Suo et al. 2021), the possibility that these toxic flatworms share a common symbiotic bacterial flora cannot be ruled out. Further research progress on remaining questions is expected in the future.
On the other hand, it is known that the components of TTX analogs are different between marine and terrestrial TTX-bearing organisms, suggesting that the biosynthetic pathways of TTX are different in both environments (Kudo et al. 2016; Ueyama et al. 2018; Sato et al. 2021). These implicate the possibility of inferring prey species comparing compositions of TTX and analogs. In the waters around the Ryukyu Islands, DNA fragments of P. multitentaculata have been detected in the intestinal contents of TTX-bearing fish, C. patoca and Y. criniger, and in the water itself at their collection site (Itoi et al. 2020a; Ito et al. 2022). In the present study, a toxification experiment, wherein cultured T. alboplumbeus were fed with P. multitentaculata eggs, also reproduced the composition pattern of TTX and analogs in wild T. alboplumbeus juveniles collected from the Ryukyu Islands. Takifugu alboplumbeus is widely distributed in the Japanese Archipelago including the Ryukyu Islands (Takagi et al. 2019), and can be made nontoxic in cultured individuals (Matsui et al. 1982), making it an appropriate model for toxification with TTX. The experiment for intramuscular administration of TTX and its analogs in T. alboplumbeus demonstrated that transformation among TTX and its analogs were not observed (Kono et al. 2008). Similar experiments in this study showed that transformation between TTX and 5,6,11-trideoxyTTX did not occur in the body of T. alboplumbeus. Since Planocera sp. has a similar TTX and analog composition to those of P. multitentaculata and P. reticulata, it is highly likely that Planocera sp. is also involved in the toxification of TTX-bearing fish in the Ryukyu Islands.
The present study on toxification using TTX-bearing planocerid egg plates leaves unanswered why the signal intensities of TTX and analogs, especially 5,6,11-trideoxyTTX, were significantly enhanced in C. patoca, but not in Y. criniger, contrary to prior expectations. It has been reported that pufferfish juveniles accumulated 2- to fivefold more TTX than 5,6,11-trideoxyTTX, regardless of collection site or species, and that the TTX/5,6,11-trideoxyTTX ratios in pufferfish juveniles were reproduced in the toxification experiment using the P. multitentaculata egg plates (Ito et al. 2023). Further, the ratio of TTX to 5,6,11-trideoxyTTX varied among Y. criniger populations collected from various sampling locations (Ito et al. 2022). Although it was thought that the difference in composition of TTX and analogs between pufferfish and toxic goby might be the result of different compositions in the prey species and/or differing uptake capacity of TTX and analogs between the two fish groups (Ito et al. 2022). This study could not reach a conclusion on this issue.
However, all of the TTX-bearing planocerids, in this study, had similar compositions of TTX and analogs, leaving the possibility that Y. criniger accumulates TTX and analogs by preying on TTX-bearing organisms other than toxic flatworms. For example, ribbonworms including Cephalothrix simula, which possess large amounts of TTX (Miyazawa et al. 1988; Asakawa et al. 2000, 2013; Vlasenko et al. 2018; Malykin et al. 2021), might be potential TTX sources for TTX-bearing fish, because C. cf. simula possesses TTX throughout its life history including planktonic larval stages (Malykin et al. 2023). In the future, we plan to examine the intestinal contents of various TTX-bearing organisms to clarify the missing link between TTX-bearing prey and predators.
Data Availability
No datasets were generated or analysed during the current study.
References
Adachi M, Sakakibara R, Satake Y, Isobe M, Nishikawa T (2014) Synthesis of 5,6,11-trideoxytetrodotoxin. Chem Lett 43:1719–1721
Antonelli P, Salerno B, Bordin P, Peruzzo A, Orsini M, Arcangeli G, Barco L, Losasso C (2022) Tetrodotoxin in live bivalve mollusks from Europe: is it to be considered an emerging concern for food safety? Compr Rev Food Sci Food Saf 21:719–737
Asakawa M, Toyoshima T, Shida Y, Noguchi T, Miyazawa K (2000) Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 38:763–773
Asakawa M, Ito K, Kajihara H (2013) Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 5:376–395
Bane V, Lehane M, Dikshit M, O’Riordan A, Furey A (2014) Tetrodotoxin: chemistry, toxicity, source, distribution and detection. Toxins 6:693–755
Biessy L, Boundy MJ, Smith KS, Harwood DT, Hawes I, Wood SA (2019) Tetrodotoxin in marine bivalves and edible gastropods: a mini-review. Chemosphere 236:124404
Donath A, Jühling F, Al-Arab M, Bernhart SH, Reinhardt F, Stadler PF, Middendorf M, Bernt M (2019) Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res 47:10543–10552
EFSA Contam Panel, Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Nebbia CS, Oswald IP, Rose M, Roudot A-C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Arnich N, Benford D, Botana L, Viviani B, Arcella D, Binaglia M, Horvath Z, Steinkellner H, van Manen M, Petersen A (2017) Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J 15:e04752
Honda S, Arakawa O, Takatani T, Tachibana K, Yagi M, Tanigawa A, Noguchi T (2005) Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi 71:815–820
Ito M, Furukawa R, Yasukawa S, Sato M, Oyama H, Okabe T, Suo R, Sugita H, Takatani T, Arakawa O, Adachi M, Nishikawa T, Itoi S (2022) Local differences in the toxin amount and composition of tetrodotoxin and related compounds in pufferfish (Chelonodon patoca) and toxic goby (Yongeichthys criniger) juveniles. Toxins 14:150
Ito M, Shirai K, Oyama H, Yasukawa S, Asano M, Kihara M, Suo R, Sugita H, Nakahigashi R, Adachi M, Nishikawa T, Itoi S (2023) Geographical differences in the composition of tetrodotoxin and 5,6,11-trideoxytetrodotoxin in Japanese pufferfishes and their origins. Chemosphere 336:139214
Itoi S, Kozaki A, Komori K, Tsunashima T, Noguchi S, Kawane M, Sugita H (2015) Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: implications for TTX accumulation in the pufferfish. Toxicon 108:141–146
Itoi S, Ueda H, Yamada R, Takei M, Sato T, Oshikiri S, Wajima Y, Ogata R, Oyama H, Shitto T, Okuhara K, Tsunashima T, Sawayama E, Sugita H (2018) Including planocerid flatworms in the diet effectively toxifies the pufferfish Takifugu niphobles. Sci Rep 8:12302
Itoi S, Sato T, Takei M, Yamada R, Ogata R, Oyama H, Teranishi S, Kishiki A, Wada T, Noguchi K, Abe M, Okabe T, Akagi H, Kashitani M, Suo R, Koito T, Takatani T, Arakawa O, Sugita H (2020a) The planocerid flatworm is a main supplier of toxin to tetrodotoxin-bearing fish juveniles. Chemosphere 249:126217
Itoi S, Tabuchi S, Abe M, Ueda H, Oyama H, Ogata R, Okabe T, Kishiki A, Sugita H (2020b) Difference in tetrodotoxin content between two sympatric planocerid flatworms, Planocera multitentaculata and Planocera reticulata. Toxicon 173:57–61
Jang JH, Lee JS, Yotsu-Yamashita M (2010) LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar Drugs 8:1049–1058
Kashitani M, Okabe T, Oyama H, Noguchi K, Yamazaki H, Suo R, Mori T, Sugita H, Itoi S (2020) Taxonomic distribution of tetrodotoxin in acotylean flatworms (Polycladida: Platyhelminthes). Mar Biotechnol 22:805–811
Katikou P, Gokbulut C, Kosker AR, Campàs M, Ozogul F (2022) An updated review of tetrodotoxin and its peculiarities. Mar Drugs 20:47
Kono M, Matsui T, Furukawa K, Takase T, Yamamori K, Kaneda H, Aoki D, Jang J-H, Yotsu-Yamashit M (2008) Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 52:714–720
Kudo Y, Yotsu-Yamashita M (2019) Isolation and biological activity of 8-epitetrodotoxin and the structure of a possible biosynthetic shunt product of tetrodotoxin, Cep-226A, from the newt Cynops ensicauda popei. J Nat Prod 82:1656–1663
Kudo Y, Finn J, Fukushima K, Sakugawa S, Cho Y, Konoki K, Yotsu-Yamashita M (2014a) Isolation of 6-deoxytetrodotoxin from the pufferfish, Takifugu pardalis, and a comparison of the effects of the C-6 and C-11 hydroxy groups of tetrodotoxin on its activity. J Nat Prod 77:1000–1004
Kudo Y, Yamashita Y, Mebs D, Cho Y, Konoki K, Yasumoto T, Yotsu-Yamashita M (2014b) C5–C10 directly bonded tetrodotoxin analogues: possible biosynthetic precursors of tetrodotoxin from newts. Angew Chem Int Ed 53:14546–14549
Kudo Y, Yasumoto T, Mebs D, Cho Y, Konoki K, Yotsu-Yamashita M (2016) Cyclic guanidine compounds from toxic newts support the hypothesis that tetrodotoxin is derived from a monoterpene. Angew Chem Int Ed 55:8728–8731
Kudo Y, Hanifin CT, Kotaki Y, Yotsu-Yamashita M (2020) Structures of N-hydroxy-type tetrodotoxin analogues and bicyclic guanidinium compounds found in toxic newts. J Nat Prod 83:2706–2717
Kudo Y, Hanifin CT, Yotsu-Yamashita M (2021) Identification of tricyclic guanidino compounds from the tetrodotoxin-bearing newt Taricha granulosa. Org Lett 23:3513–3517
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Magarlamov TY, Melnikova DI, Chernyshev AV (2017) Tetrodotoxin-producing bacteria: detection, distribution and migration of the toxin in aquatic systems. Toxins 9:166
Malykin GV, Chernyshev AV, Magarlamov TY (2021) Intrabody tetrodotoxin distribution and possible hypothesis for its migration in ribbon worms Cephalothrix cf. simula (Palaeonemertea Nemertea). Mar Drugs 19:494
Malykin GV, Velansky PV, Melnikova DI, Magarlamov TY (2023) Tetrodotoxins in larval development of ribbon worm Cephalothrix cf. simula (Palaeonemertea, Nemertea). Mar Biotechnol 25:918–934
Matsui T, Hamada S, Konosu S (1981) Difference in accumulation of puffer fish toxin and crystalline tetrodotoxin in the puffer fish, Fugu rubripes rubripes. Bull Jpn Soc Sci Fish 47:535–537
Matsui T, Sato H, Hamada S, Shimizu C (1982) Comparison of toxicity of the cultured and wild puffer fish Fugu niphobles. Bull Jpn Soc Sci Fish 48:253
Miyazaki K, Suo R, Itoi S, Hirota J, Adachi M, Miyasaka T, Nishikawa T, Yokoyama T, Sato S, Takada K (2024) 5, 6, 11-trideoxy tetrodotoxin attracts tiger puffer Takifugu rubripes. Toxicon 237
Miyazawa K, Jeon JK, Maruyama J, Noguchi T, Ito K, Hashimoto K (1986) Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 24:645–650
Miyazawa K, Higashiyama M, Ito K, Noguchi T, Arakawa O, Shida Y, Hashimoto K (1988) Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 26:897–864
Narahashi T (2001) Pharmacology of tetrodotoxin. J Toxicol Toxin Rev 20:67–84
Noguchi T, Arakawa O (2008) Tetrodotoxin - distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar Drugs 6:220–242
Noguchi T, Arakawa O, Takatani T (2006) TTX accumulation in pufferfish. Comp Biochem Physiol Part D 1:145–152
Okabe T, Oyama H, Kashitani M, Ishimaru Y, Suo R, Sugita H, Itoi S (2019) Toxic flatworm egg plates serve as a possible source of tetrodotoxin for pufferfish. Toxins 11:402
Okabe T, Saito R, Yamamoto K, Watanabe R, Kaneko Y, Yanaoka M, Furukoshi S, Yasukawa S, Ito M, Oyama H, Suo R, Suzuki M, Takatani T, Arakawa O, Sugita H, Itoi S (2021) The role of toxic planocerid flatworm larvae on tetrodotoxin accumulation in marine bivalves. Aquat Toxicol 237:105908
Oyama H, Ito M, Suo R, Goto-Inoue N, Morisasa M, Mori T, Sugita H, Mori T, Nakahigashi R, Adachi M, Nishikawa T, Itoi S (2022) Changes in tissue distribution of tetrodotoxin and its analogues in association with maturation in the toxic flatworm, Planocera multitentaculata. Mar Biotechnol 24:1158–1167
Rambla-Alegre M, Reverté L, Del Río V, de la Iglesia P, Palacios O, Flores C, Caixach J, Campbell K, Elliott CT, Izquierdo-Muñoz A, Campàs M, Diogène J (2017) Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ Res 158:1–6
Ritson-Williams R, Yotsu-Yamashita M, Paul VJ (2006) Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc Natl Acad Sci USA 103:3176–3179
Salvitti L, Wood SA, Taylor DI, McNabb P, Cary SC (2015) First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; a source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 95:23–29
Sato S, Kawaura R, Togashi K, Mizusawa N, Ko Yasumoto K, Takada K, Amano M, Watabe S (2021) De novo accumulation of tetrodotoxin and its analogs in pufferfish and newt and dosage-driven accumulation of toxins in newt: tissue distribution and anatomical localization. J Mar Sci Eng 9:1004
Stokes AN, Ducey PK, Neuman-Lee L, Hanifin CT, French SS, Pfrender ME, Brodie ED III, Brodie ED Jr (2014) Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 9
Suo R, Kashitani M, Oyama H, Adachi M, Nakahigashi R, Sakakibara R, Nishikawa T, Sugita H, Itoi S (2021) First detection of tetrodotoxins in the cotylean flatworm Prosthiostomum trilineatum. Mar Drugs 19:40
Suo R, Tanaka M, Oyama H, Kojima Y, Yui K, Sakakibara R, Adachi M, Nishikawa T, Sugita H, Itoi S (2022) Tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 216:169–173
Suo R, Tanaka M, Asano M, Nakahigashi R, Adachi M, Nishikawa N, Ogiso S, Matsubara H, Suzuki N, Itoi S (2024) Distribution of tetrodotoxin and its analogues in the toxic flatworm Planocera multitentaculata from the Honshu Island, Japan. Fish Sci 90:319–326
Takagi M, Toyama K, Yamada Y, Sakai H (2019) Genetic difference of grass puffer Takifugu alboplumbeus population in the Okinawajima Island, Japan. Fauna Ryukyuana 49:1–11
Tanu MB, Mahmud Y, Arakawa O, Takatani T, Kajihara H, Kawatsu K, Hamano Y, Asakawa M, Miyazawa K, Noguchi T (2004) Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (nemertea: anopla: palaeonemertea: cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 44:515–520
Ueda H, Itoi S, Sugita H (2018) TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands. Japan Mar Drugs 16:37
Ueyama N, Sugimoto K, Kudo Y, Onodera KI, Cho Y, Konoki K, Nishikawa T, Yotsu-Yamashita M (2018) Spiro bicyclic guanidino compounds from pufferfish: Possible biosynthetic intermediates of tetrodotoxin in marine environments. Chemistry 24:7250–7258
Vlasenko AE, Magarlamov TY (2020) Tetrodotoxin and its analogues in Cephalothrix cf. simula (nemertea: palaeonemertea) from the Sea of Japan (Peter the Great Gulf): intrabody distribution and secretions. Toxins 12:745
Vlasenko AE, Magarlamov TY (2022) Tetrodotoxins in ribbon worms Cephalothrix cf. simula and Kulikovia alborostrata from Peter the Great Bay. Sea of Japan. Toxins 15:16
Vlasenko AE, Velansky PV, Chernyshev AV, Kuznetsov VG, Magarlamov TY (2018) Tetrodotoxin and its analogues profile in nemertean species from the Sea of Japan. Toxicon 156:48–51
Yamada R, Tsunashima T, Takei M, Sato T, Wajima Y, Kawase M, Oshikiri S, Kajitani Y, Kosoba K, Ueda H, Abe K, Itoi S, Sugita H (2017) Seasonal changes in the tetrodotoxin content of the flatworm Planocera multitentaculata. Mar Drugs 15:56
Yasukawa S, Shirai K, Namigata K, Ito M, Tsubaki M, Oyama H, Fujita Y, Okabe T, Suo R, Ogiso S, Watabe Y, Matsubara H, Suzuki N, Hirayama M, Sugita H, Itoi S (2023) Tetrodotoxin detection in Japanese bivalves: toxification status of scallops. Mar Biotechnol 25:666–676
Yonezawa R, Itoi S, Igarashi Y, Yoshitake K, Oyama H, KinoshitaS SR, Yokobori S, Sugita H, Asakawa S (2020) Characterization and phylogenetic position of two sympatric sister species of toxic flatworms Planocera multitentaculata and Planocera reticulata (Platyhelminthes: Acotylea). Mitochondr DNA B 5:2352–2354
Acknowledgements
We thank Prof. Toshio Nishikawa (Nagoya University) and Dr. Masaatsu Adachi (Tohoku University) for providing chemically synthesized 5,6,11-trideoxyTTX. We express sincere thanks to Ms. Masami Hashimoto and staff of Diving Shop NANA for collecting samples, and to Taiki Okabe, Niigata Prefectural Kaiyo High School, for providing the non-toxic pufferfish Takifugu alboplumbeus.
Funding
This study was supported in part by the Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (JSPS) (19H00954, 23H00347, S.I., S.A.) and Grant-in-Aid for JSPS Fellows from JSPS (20J20428, H.O.; 22KJ0783, R.Y.), Grant-in-aid for Young Scientific Research from JSPS (20K15606, R.S.), the Research Grant of Nihon University (2023–2024, S.I.), and the JST ACT-X (JPMJAX22BC, R.S.).
Author information
Authors and Affiliations
Contributions
Study conception and design: Hiroyuki Ueda, Rei Suo, Shuichi Asakawa, and Shiro Itoi. Sample collection: Hiroyuki Ueda, Masaaki Ito, Ryo Yonezawa, Kentaro Hayashi, Maho Kashitani, Hikaru Oyama, Kyoko Shirai, Rei Suo, and Shiro Itoi. TTX extraction and LC–MS/MS analysis: Hiroyuki Ueda, Masaaki Ito, Taiga Tomonou, Maho Kashitani, Hikaru Oyama, Kyoko Shirai, and Rei Suo. NGS analysis: Hiroyuki Ueda, Ryo Yonezawa, Kentaro Hayashi, Kazutoshi Yoshitake, Shigeharu Kinoshita, and Shuichi Asakawa. Manuscript drafting: Hiroyuki Ueda, and Masaaki Ito. Critical revision: Shiro Itoi. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The authors declare that this manuscript complies with the Springer Ethical Guidelines for Journal Publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ueda, H., Ito, M., Yonezawa, R. et al. Japanese Planocerid Flatworms: Difference in Composition of Tetrodotoxin and Its Analogs and the Effects of Ingestion by Toxin-Bearing Fishes in the Ryukyu Islands, Japan. Mar Biotechnol 26, 500–510 (2024). https://doi.org/10.1007/s10126-024-10312-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-024-10312-0