Abstract
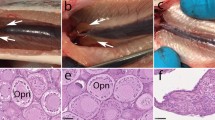
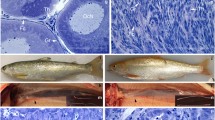
In germ cell transplantation experiments, the use of sterile recipients that do not produce their own gametes is an important prerequisite. Triploidization and dnd gene knockdown (KD) methods have been widely used to produce sterile fish. However, triploidization does not produce complete sterility in some fish species, and gene KD is labor and time intensive since it requires microinjection into individual fertilized eggs. To overcome these problems, in this study, we generated homozygous mutants of the dead end (dnd) gene in rainbow trout (Oncorhynchus mykiss) using the clustered regulatory interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) system, analyzed their reproductive capacity, and evaluated their suitability as recipients for germ cell transplantation. By crossing F1 heterozygous mutants produced from founders subjected to genome editing, an F2 generation consisting of approximately 1/4 homozygous knockout mutants (dnd KO) was obtained. The dnd KO hatchlings retained the same number of primordial germ cells (PGCs) as the wild-type (WT) individuals, after which the number gradually decreased. At 1 year of age, germ cells were completely absent in all analyzed individuals. To evaluate the dnd KO individuals as recipients for germ cell transplantation, germ cells prepared from donor individuals were transplanted into the abdominal cavity of dnd KO hatchlings. These cells migrated to the recipient gonads, where they initiated gametogenesis. The mature recipient individuals produced only donor-derived sperm and eggs in equivalent numbers to WT rainbow trout. These results indicate that dnd KO rainbow trout are suitable recipient candidates possessing a high capacity to nurse donor-derived germ cells.






Similar content being viewed by others
Data Availability
All plasmid DNA used in this study is available upon reasonable request.
References
Ansai S, Kinoshita M (2014) Targeted mutagenesis using CRISPR/Cas system in medaka. Biol Open 3:362–371
Baloch AR, Franěk R, Saito T, Pšenička M (2021) Dead-end (dnd) protein in fish—a review. Fish Physiol Biochem 47:777–784
Chen J, Zhang X, Wang T, Li Z, Guan G, Hong Y (2012) Efficient detection, quantification and enrichment of subtle allelic alterations. DNA Res 19:423–433
Franěk R, Kašpar V, Shah MA, Gela D, Pšenička M (2021) Production of common carp donor-derived offspring from goldfish surrogate broodstock. Aquaculture 534:736252
Fujii W, Kawasaki K, Sugiura K, Naito K (2013) Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Res 41:e187
Fujimoto T, Nishimura T, Goto-Kazeto R, Kawakami Y, Yamaha E, Arai K (2010) Sexual dimorphism of gonadal structure and gene expression in germ cell-deficient loach, a teleost fish. Proc Natl Acad Sci U S A 107:17211–17216
Goto R, Saito T, Takeda T, Fujimoto T, Takagi M, Arai K, Yamaha E (2012) Germ cells are not the primary factor for sexual fate determination in goldfish. Dev Biol 370:98–109
Gross-Thebing T, Yigit S, Pfeiffer J, Reichman-Fried M, Bandemer J, Ruckert C, Rathmer C, Goudarzi M, Stehling M, Tarbashevich K, Seggewiss J, Raz E (2017) The vertebrate protein dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev Cell 43:704–715
Güralp H, Skaftnesmo KO, Kjærner-Semb E, Straume AH, Kleppe L, Schulz RW, Edvardsen RB, Wargelius A (2020) Rescue of germ cells in dnd crispant embryos opens the possibility to produce inherited sterility in Atlantic salmon. Sci Rep 10:18042
Hamasaki M, Takeuchi Y, Miyaki K, Yoshizaki G (2013) Gonadal development and fertility of triploid grass puffer Takifugu niphobles induced by cold shock treatment. Mar Biotechnol 15:133–144
Hamasaki M, Takeuchi Y, Yazawa R, Yoshikawa S, Kadomura K, Yamada T, Miyaki K, Kikuchi K, Yoshizaki G (2017) Production of tiger puffer Takifugu rubripes offspring from triploid grass puffer Takifugu niphobles parents. Mar Biotechnol 19:579–591
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Iwasaki-Takahashi Y, Shikina S, Watanabe M, Banba A, Yagisawa M, Takahashi K, Fujihara R, Okabe T, Valdez-Jr DM, Yamauchi A, Yoshizaki G (2020) Production of functional eggs and sperm from in vitro-expanded type A spermatogonia in rainbow trout. Commun Biol 3:308
Katayama N, Kume S, Hattori-Ihara S, Sadaie S, Hayashi M, Yoshizaki G (2016) Germ cell-specific excision of loxP-flanked transgenes in rainbow trout Oncorhynchus mykiss. Biol Reprod 94:79
Kawamura W, Tani R, Yahagi H, Kamio S, Morita T, Takeuchi Y, Yazawa R, Yoshizaki G (2020) Suitability of hybrid mackerel (Scomber australasicus × S. japonicus) with germ cell-less sterile gonads as a recipient for transplantation of bluefin tuna germ cells. Gen Comp Endocrinol 295:113525
Kobayashi T, Nakamura M, Kajiura-Kobayashi H, Young G, Nagahama Y (1998) Immunolocalization of steroidogenic enzymes (P450scc, P450c17, P450arom, and 3beta-HSD) in immature and mature testes of rainbow trout (Oncorhynchus mykiss). Cell Tissue Res 292:573–577
Kurokawa HD, Saito S, Nakamura Y, Katoh-Fukui K, Ohta T, Baba K, Morohashi I, Tanaka M (2007) Germ cells are essential for sexual dimorphism in the medaka gonad. Proc Natl Acad Sci USA 104:16958–16963
Lee S, Iwasaki Y, Shikina S, Yoshizaki G (2013) Generation of functional eggs and sperm from cryopreserved whole testes. Proc Natl Acad Sci USA 110:1640–1645
Lee S, Seki S, Katayama N, Yoshizaki G (2015) Production of viable trout offspring derived from frozen whole fish. Sci Rep 5:16045
Li Q, Fujii W, Naito K, Yoshizaki G (2017) Application of dead end-knockout zebrafish as recipients of germ cell transplantation. Mol Reprod Dev 84:1100–1111
Linhartová Z, Saito, Kašpar V, Rodina M, Prášková E, Hagihara S, Pšenička M (2015) Sterilization of sterlet Acipenser ruthenus by using knockdown agent, antisense morpholino oligonucleotide, against dead end gene. Theriogenology 84:1246–1255
Nakajima S, Hayashi M, Kouguchi T, Yamaguchi K, Miwa M, Yoshizaki G (2014) Expression patterns of gdnf and gfrα1 in rainbow trout testis. Gene Expr Patterns 14:111–120
Octavera A, Yoshizaki G (2019) Production of donor-derived offspring by allogeneic transplantation of spermatogonia in Chinese rosy bitterling. Biol Reprod 100:1108–1117
Octavera A, Yoshizaki G (2020) Production of Chinese rosy bitterling offspring derived from frozen and vitrified whole testis by spermatogonial transplantation. Fish Physiol Biochem 46:1431–1442
Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G (2007) Surrogate broodstock produces salmonids. Nature 317:1517
Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G (2006) Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci USA 103:2725–2729
Saito T, Goto-Kazeto R, Arai K, Yamaha E (2008) Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 78:159–166
Sawatari E, Shikina S, Takeuchi T, Yoshizaki G (2007) A novel transforming growth factor- β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial preliferation in rainbow trout (Oncorhynchus mykiss). Develop Biol 301:266–275
Slanchev KJ, Stebler G, Cueva-Méndez RE (2005) Development without germ cells: the role of the germ line in zebrafish sex differentiation. Proc Natl Acad Sci USA 102:4074–4079
Takeuchi Y, Yatabe T, Yoshikawa Y, Ino Y, Kabeya N, Yazawa R, Yoshizaki G (2016) Production of functionally sterile triploid Nibe croaker Nibea mitsukurii induced by cold-shock treatment with special emphasis on triploid aptitude as surrogate broodstock. Aquaculture 494:45–56
Takeuchi Y, Yoshizaki G, Kobayashi T, Takeuchi T (2002) Mass isolation of primordial germ cells from transgenic rainbow trout carrying the green fluorescent protein gene driven by the vasa gene promoter. Biol Reprod 69:1087–1092
Takeuchi Y, Yoshizaki G, Takeuchi T (1999) Green fluorescent protein as a cell-labeling tool and a reporter of gene expression in transgenic rainbow trout. Mar Biotechnol 1:448–457
Tyler CR, Pottinger TG, Santos E, Sumpter JP, Price SA, Brooks S, Nagler JJ (1996) Mechanisms controlling egg size and number in the rainbow trout, Oncorhynchus mykiss. Biol Reprod 54:8–15
Wargelius A, Leininger S, Skaftnesmo KO, Kleppe L, Andersson E, Taranger GL, Schulz RW, Edvardsen RB (2016) Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep 6:21284
Weidinger G, Stebler J, Slanchev K, Dumstrei K, Wise C, Lovell-Badge R, Thisse C, Thisse B, Raz E (2003) Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr Biol 13:1429–1434
Yoshikawa H, Ino Y, Kishimoto K, Koyakumaru H, Saito T, Kinoshita M, Yoshiura Y (2020) Induction of germ cell-deficiency in grass puffer by dead end 1 gene knockdown for use as a recipient in surrogate production of tiger puffer. Aquaculture 526:735385
Yoshikawa H, Xu D, Ino Y, Yoshino T, Hayashida T, Wang J, Yazawa R, Yoshizaki G, Takeuchi Y (2018) Hybrid sterility in fish caused by mitotic arrest of primordial germ cells. Genetics 209:507–521
Yoshizaki G, Lee S (2018) Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res 29:103–110
Yoshizaki G, Takashiba K, Shimamori S, Fujinuma K, Shikina S, Okutsu T, Kume S, Hayashi M (2016) Production of germ cell-deficient salmonids by dead end gene knockdown, and their use as recipients for germ cell transplantation. Mol Reprod Dev 83:298–311
Yoshizaki G, Takeuchi Y, Sakatani S, Takeuchi T (2000) Germ cell-specific expression of green fluorescent protein in rainbow trout under the control of the rainbow trout vasa-like gene promoter. Int J Dev Biol 44:323–326
Yoshizaki G, Yazawa R (2019) Application of surrogate broodstock technology in aquaculture. Fish Sci 85:429–437
Funding
This study was partly sponsored by MEXT (18H05545 and 20H00430) and JST (JPMJMI21C1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujihara, R., Katayama, N., Sadaie, S. et al. Production of Germ Cell-Less Rainbow Trout by dead end Gene Knockout and their Use as Recipients for Germ Cell Transplantation. Mar Biotechnol 24, 417–429 (2022). https://doi.org/10.1007/s10126-022-10128-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-022-10128-w